|
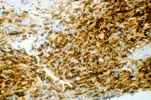
Posttransplant Lymphoproliferative Disorders Marked progress has been made in recent years in the area of pancreas and kidney-pancreas transplantation mostly due to the introduction of new immunosuppressive drugs. Associated with the use of powerful immunosuppressive regimens is an increased risk for development of lymphoproliferative disorders. The incidence of EBV-related PTLD in pancreas and pancreas-kidney transplant recipients has ranged from 5.2 (per 100 person year) to 12%. At the University of Maryland pancreas transplant program the incidence of PTLD has been 2.2%. This number is similar to the overall incidence of PTLD in other solid organ transplant recipients, which is reported to be about 2%.
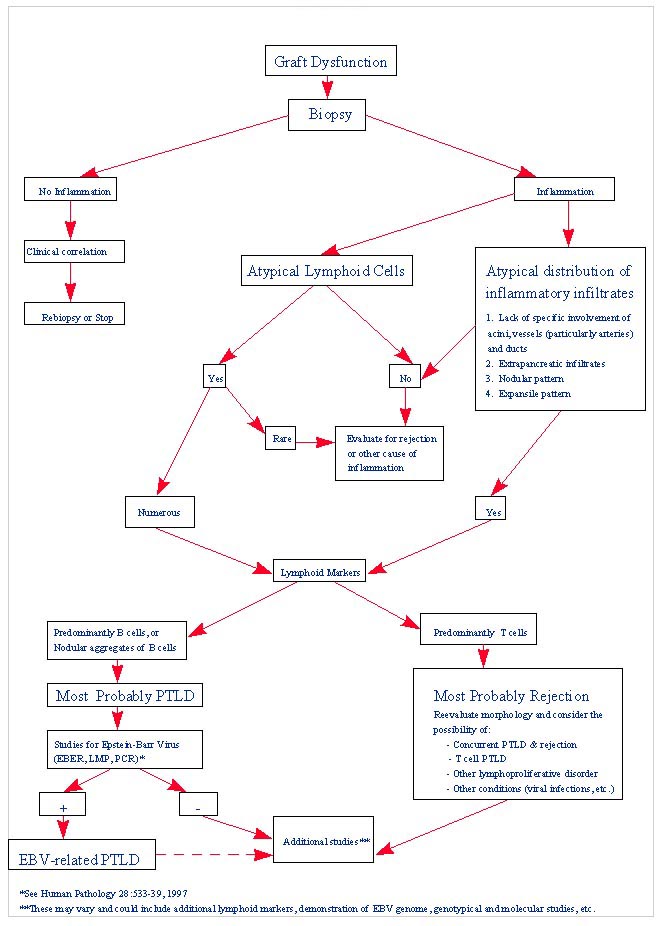
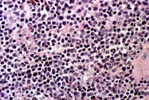
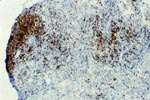
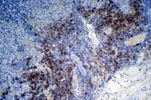
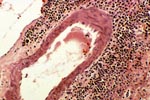
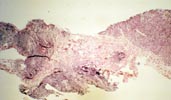
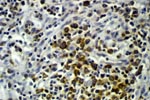
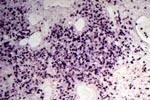
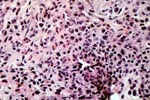
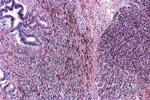
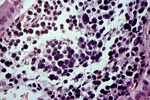
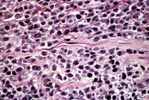
PTLD commonly involves the graft itself in addition to affecting extranodal locations such as the gastrointestinal tract and the brain. In allograft biopsies the main differential diagnosis of PTLD includes the most severe forms of acute allograft rejection. Dense inflammatory infiltrates are common in acute rejection. These infiltrates are usually mixed and include variable proportions of activated lymphocytes (immunoblasts) that may show significant cytologic atypia. Reduction in immunosuppression is of paramount importance in the treatment of PTLD, whereas misdiagnosis of these lesions as rejection reactions will lead to the contrary. For the same reasons, delay in the correct diagnosis of PTLD may lead to potentially life-threatening progression of the disease. The mortality that may be associated with PTLD is particularly unacceptable in the situation of elective organ transplantation (i.e. kidney, pancreas). The situation is different in the case of patients with liver or heart/lung transplants in which the preservation of graft function is of vital importance for the recipient. Helpful features useful in the differentiation of rejection and PTLD in pancreas allograft tissue samples are described below. Features of rejection Acute allograft rejection is characterized by mixed inflammatory infiltrates composed of small and larger/activated lymphocytes, eosinophils, rare plasma cells and neutrophils. The infiltrates consist of a predominant (>75%) population of T cells (CD3 and UCHL-1 positive). B cells are seen scattered among the rest of the infiltrates (<15% in most samples). Small tight clusters of B cells measuring no more than 200-300 m in diameter are sometimes identified; nodular aggregates of B cells are not seen in rejection. Eosinophils in variable proportions (1-10%) are common. Mature appearing plasma cells (2-5% of infiltrates) are present in most specimens. Neutrophils are present in areas showing acinar necrosis and in samples with vascular rejection. Immunoperoxidase stains for kappa and lambda immunoglobulin light chains demonstrate a mixture of scattered kappa and lambda positive cells. Atypical immunoblasts and plasmacytoid cells displaying nuclear enlargement and hyperchromasia usually represent less than 10% of the inflammatory cells. The inflammation in rejection involves the fibrous septa with cuffing of veins and often displays associated venous endotheliitis; the infiltrates permeate into the acinar parenchyma and relate tightly to the acini causing acinar cell damage. In the inflamed areas, often there is inflammation of the interlobular ducts with associated reactive and degenerative changes in the epithelial cells. Islets and nerves are involved randomly and proportionally to the overall degree of inflammatory infiltrates. In samples showing rejection the infiltrates involve predominantly the exocrine parenchyma with only minimal sparse mononuclear inflammatory infiltrates consisting of mature appearing T lymphocytes occasionally seen in the peripancreatic fat. Arterial endotheliitis and/or arteritis are typical of the more severe forms of rejection (Grades IV and V). Patchy or confluent necrosis is characteristic of severe rejection (Grade V). In situ hybridization for EBER is negative or shows positivity in very rare cells in typical cases of rejection. The same is true for stains for LMP. Features of PTLD Samples with the monomorphic types of lymphoma show infiltration by sheets of monomorphic atypical immunoblasts of B cell immunophenotype. Immunoglobulin light chain restriction may be identified. Extensive parenchymal infiltration and geographic areas of necrosis are common in these cases. Samples involved by polymorphic PTLD, show mixed infiltrates with variable proportions of plasmacytoid cells with atypical markedly hyperchromatic nuclei. Rare Reed-Sternberg-like cells may be seen. The atypical cells range from 10% to more than 70%, depending on the presence or absence of concurrent rejection. In the earlier biopsies with PTLD CD3 and UCHL-1 stains may demonstrate a significant proportion of T cells (40-50%) as well as morphological features of concurrent acute rejection. Typical PTLD however, shows a minority of T cell (<:25%). In the early forms of PTLD, CD20 stains show in addition to scattered positive cells, irregularly shaped nodular proliferations of B cells expanding from the septal areas. Immunoglobulin light chain restriction may be demonstrated in patients with polymorphic B cell hyperplasia/lymphoma. Significant numbers of neutrophils are only seen in areas of necrosis. Eosinophils are commonly seen in samples with concurrent rejection. In the samples with PTLD a significant proportion of the atypical cells is positive for EBER and about 5-10% of the cells are positive for LMP-1. In contrast to rejection which shows consistent septal inflammation with associated proportional spotty or severe involvement of the acinar parenchyma, in PTLD the infiltrates are randomly distributed with large areas of parenchyma completely free of infiltrates. In the samples with early involvement by PTLD the infiltrates are seen predominantly in septa or in peripancreatic tissues. Geographic areas of coagulation necrosis are present in PTLD, however, this is not common in samples with early involvement. In the inflamed but viable areas the acini and ducts do not show significant damage as typically seen in rejection. All sizes of veins are consistently infiltrated by the atypical B cells; associated lifting and damage of the endothelium similar to the rejection associated endotheliitis is common. Nerves and adjacent soft tissues are usually extensively infiltrated in the pancreatectomies involved by PTLD. Concurrent vascular rejection is not uncommon in samples with PTLD; the involved arteries are permeated by a predominant population of small T cells and show endotheliitis and/or arteritis. In the absence of rejection the arterial walls are however, consistently resistant to permeation by the atypical B-cell infiltrates. EBV related PTLD include a wide range of processes from benign hyperplastic to overtly malignant lymphoid proliferations. On the most benign end of the spectrum (plasmacytic hyperplasia) the patients present with generalized type of symptoms and lymphadenopathy rather than with graft dysfunction and therefore graft biopsies usually play no role in the diagnostic work up. Graft involvement is not unusual in the other forms of PTLD (polymorphic B cell hyperplasia/ lymphoma, immunoblastic lymphoma), as previously reported in the kidney. The vast majority of PTLD are of B cell origin whereas acute allograft rejection is characterized by a predominant population of T cells. In the occasional patients who present with acute allograft dysfunction and a biopsy showing atypical infiltrates questionable for PTLD, we have found that for screening purposes immunoperoxidase stains for B and T cell markers are extremely helpful. Based on the H&E morphological findings and these immunohistochemical stains a preliminary diagnosis can be rendered in a matter of hours. Dense aggregates of B cells were never seen in biopsies from patients with pure rejection even in the presence of heavy and atypical infiltrates. Significant overlap may however occur in some cases, particularly since these patients often present with concurrent refractory rejection requiring higher than usual immunosuppressive therapy. In all cases the assessment needs to be both quantitative as well as qualitative taking particularly in account the degree of cytologic atypia present in the sample. The determination of immunoglobulin light chain restriction when present leads to unequivocal diagnosis of a lymphoproliferative disorder, but is not necessarily helpful in the differential diagnosis between acute rejection and PTLD since it is absent in a significant proportion of the latter. An accurate diagnosis of EBV-related PTLD requires the confirmation of the presence of EBV genome with in situ hybridization for EBER or with PCR studies.
Relevant References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||