|
Failed Grafts 

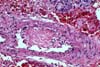
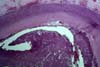
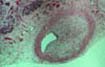
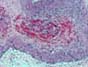

Although excellent rates of 1-year patient and graft survival have
been achieved in recent years for all
types of technically successful pancreas transplants (SPK, PTA, PAK) a significant number of grafts are still lost in the early post-implantation period, due to a variety
of surgical complications. Graft losses
due to leaks, bleeding, thrombosis, infections and early pancreatitis are grouped together under the
category of technical failure. Currently the number of surgical complications
is disproportionately high in comparison to the number of graft loses due to
acute rejection. Thrombosis continues to be the leading cause of
non-immunological graft loss (5.8-16.4 %) with higher rates seen in PAK and PTA
cases with enteric drainage. The pancreas has intrinsically a low blood flow compared to other solid
organs. Perioperative inflammation and edema, as well as microvascular and
endothelial damage relating to donor factors and organ preservation, all
contribute to further compromise blood flow in the early post-transplant period
leading to thrombosis. Correspondingly
longer cold ischemia times have been associated with increased incidence of
graft thrombosis. Acute rejection is suspected to play a role in some patients with early
graft thrombosis. HLA mismatches appear
not to impact on the incidence of graft
loss due to technical failures, however, HLA mismatch does have an overall negative impact on graft survival. Graft pancreatectomies:
Guidelines for gross and histological evaluation Graft pancreatectomies usually consist of the whole pancreas and attached portion of duodenum. The latter is present in continuity either
with a loop of recipient's small intestine
or a patch of urinary bladder wall. Systematic histological evaluation of failed grafts is necessary for accurate
classification of the cause of graft
loss. Minimun histological sampling
should include cross sections of all large vessels and several sections from
the parenchyma to include an adequate number of medium sized and small vessels.
The number of histological
sections depends on each case; the most
important structures should be sampled routinely. Gross evaluation: - Large arteries and veins: evaluate for thrombosis (recent and
organized), endotheliitis, arteriris, transplant vasculopathy) - Random samples from parenchyma (viable and necrotic, usually 3-5
sections): evaluate for evidence of ischemia/pancreatitis, acute rejection, chronic rejection, presence
of infectious organisms, etc.). - Area of anastomosis: evaluate for dehiscences (leaks) - Samples from any other lesions: masses (i.e. PTLD), cysts, abscesses,
lymph nodes, etc.) Ancillary studies: - Immunoperoxidase
stains for insulin and glucagon should be perfomed to evaluate for selective
destruction of beta cells (recurrence of autoimmune destruction). These cases show near normal parenchyna with
no significant evidence of fibrosis/acinar loss (chronic rejection). - Frozen tissue for
immunfluorescence stains for immunoglobulins and complement in cases suspected to represent
hyperacute rejection. - Electron
microscopy: May be helpful in recurrence of diabetes type 1. Histological
evaluation: Based on the histological findings pancreatectomies can be broadly
classified as follows. 1)
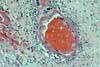
PURE VASCULAR THROMBOSIS IN AN OTHERWISE NORMAL PANCREAS In these grafts the only pathological changes
consist of recent vascular thrombosis and bland ischemic parenchymal
necrosis. There is no underlying vascular pathology or any other specific
histological change. (In early
graft loss the most important histological determination relates to blood
vessels.) The majority of these grafts (78%) are lost in
less than 48 hours after transplantation and we have never seen this pattern in
grafts lost after the first 2 weeks post-transplantation. In the case of early thrombosis, the lack of obvious histological changes associated with
the thrombosis does not rule out ultrastructural
or subtle functional damage in these organs, since older donor age and longer
cold ischemia times are associated with increased risk for early
thrombosis. 2) HYPERACUTE
ALLOGRAFT REJECTION Hyperacute rejection in the pancreas is indistinguishable from hyperacute rejection affecting other organs, and it is characterized
by necrosis of arteries and veins with
secondary massive and immediate
thrombosis and parenchymal necrosis. We
have rarely seen rapid graft loss (within 1-12 hours post-transplantation) with
extensive fibrinoid necrosis of arteries and veins with associated massive
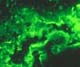
vascular thrombosis and parenchymal necrosis. Immunohistochemical studies in these cases were positive for IgG and C3 in the wall of blood vessels.
3)
ACUTE ALLOGRAFT REJECTION Graft loss secondary to acute allograft rejection
(other than hyperacute rejection) can be seen from the first week to few months
post-transplantation (in our experience
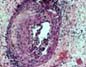
at a mean of 5.1 weeks). These cases show endotheliitis and various degrees of necrotizing arteritis (acute
rejection Grades IV-V). Graft losses
in the first months may result from a combination of infection and rejection. 4)ACUTE AND CHRONIC REJECTION Patients with with persistent (biopsy proven) acute allograft rejection can show early interstitial fibrosis
and acinar loss consistent with chronic rejection, starting in the second month
post-transplantation. In our experience graft pancreatectomies with combined
features of acute and chronic rejection
were resected at times ranging from 6 weeks to 20 months (mean 6.6 months). 5) PANCREATITIS AND
PERIPANCREATITIS Necrotizing infectious duodeno-pancreatitis with or without abscess
formation can present at variable times post-transplantation but occur often
early on (mean 3.6 months); a vaiety of
organisms can be cultured from these grafts, most commontly enterobacteria
(Enterobacter cloacae, Proteus mirabilis) and Methicillin resistant
Staphylococcus aureus (MRSA)). Fungal infections (Often Candida) and mixed
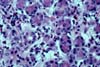
infection are not uncommon. 6) CHRONIC REJECTION These pancreatectomies show extensive interstitial fibrosis/ acinar
atrophy and transplant (obliterative) arteriopathy. This pattern can be seen after few months post-transplantation
but is more commonly seen after the second year post-transplantation (mean 28.6
months , range 4 to 81 months). Many of
these grafts do not show any significant concurrent acute rejection. Chronic rejection is the most important cause of graft loss after the
first 6 months post-transplantation. 7) POST-TRANSPLANT
LYMPHOPROLIFERATIVE DISORDER In our center allograft pancreatectomies for PTLD were performed in 4
patients in the second month post-transplantation and in one patient in month
12 (mean 5 months). The histological findings are described in the EBV section.
INCIDENCE OF
VASCULAR THROMBOSIS Recent thrombosis is seen to some degree in all cases of early graft
loss due to acute allograft rejection with vascular involvement (superimposed
on endotheliitis or arteritis) . All cases with chronic
rejection show scattered vessels with thrombosis (acute and chronic; this
typically involves medium size to small
arteries and veins. Acute vascular thrombosis in one or more large vessels can lead to
graft pancreatectomy in otherwise well
functioning allografts. In these cases the thrombosis always occurs in
abnormal blood vessels, either showing transplant arteriopathy or lesions
consistent with healing vasculitis/endotheliitis. The presence of transplant
arteriopathy (one of the histological features of chronic rejection) is
strongly associated with recent and
organized thrombosis. Correspondingly old (organized) thrombosis is seen almost
invariably in pancreatectomies with chronic rejection. As expected, progressive graft fibrosis (correlating with increasing grades of
chronic rejection) and the presence of transplant arteriopathy are directly related to the time elapsed after transplantation. Thrombosis is insignificant in the pancreatectomies performed for infectious
processes. REFERENCES Gruessner RWG, Dunn
DL, Gruessner AC, Matas AJ, Najarian JS, Sutherland DER: Recipient risk factors
have an impact on technical failure and patient and graft survival rates in
bladder-drained pancreas transplants. Transplantation
1994;57:1598-1606. Gruessner RWG,
Sutherland DER, Troppman C et al: The surgical risk of pancreas transplantation
in the Cyclosporine era: An overview. J
Am Coll Surg 1997;185:128-44. Gruessner AC and
Sutherland DER: Analysis of United States (US) and Non-US pancreas transplants
as reported to the International Pancreas Transplant Registry (IPTR) and to the
United Network for Organ Sharing (UNOS). In: Clinical Transplants 1998, pp
53-71, Cecka and Terasaki, Eds. UCLA Tissue Typing Laboratory, Los Angeles
California. Gruessner RWG,
Troppmann C, Barrou B et al: Assessment of donor and recipient risk factors on
pancreas transplant outcome. Transplant Proc 1994;26:437-8. Feitosa Tajra LC,
Dawhara M, Benchaib M, Lefrancois N, Martin X, Dubernard JM: Effect of the
surgical technique on long-term outcome of pancreas transplantation. Transpl
Int 1998;11:295-300. Morel P, Gillingham
KJ, Moudry-Munns KC, Dunn DL, Najarian
JS, Sutherland DER: Factors influencing pancreas transplant outcome: Cox
proportional hazard regression analysis of a single institution's experience
with 357 cases. Transplant Proc 1991;23:1630-33. Osaki CF, Stratta R,
Taylor RJ, Langnas AN, Bynon JS, Shaw BW: Surgical complications in solitary
pancreas and combined pancreas-kidney transplantations. Am J Surg 1992;164:546-51. Sollinger HW:
Pancreatic transplantation and vascular graft thrombosis. J Am Coll Surg
1996;182:362-3. Stratta RJ: Graft
failure after solitary pancreas transplantation. Transplant Proc 1998;30:289. Stratta RJ: Patterns
of graft loss following simultaneous kidney-pancreas transplantation. Transplant Proc 1998;30:288. Stratta RJ, Gaber
AO, Shokouh-Amiri MH, Reddy KS, Egidi MF, Grewal HP: Allograft pancreatectomy
after pancreas transplantation with systemic-bladder versus portal-enteric
drainage. Clin Transplant 1999;13:465-72. Kuo PC, Wong J,
Schweitzer EJ, Johnson LB, Lim JW, Bartlett ST: Outcome after splenic vein
thrombosis in the pancreas allograft. Transplantation 1997;64:933-5. Benedetti E,
Troppmann C, Gruessner AC, Sutherland DE, Dunn DL, Gruessner WG: Pancreas graft
loss caused by intra-abdominal infection. A risk factor for a subsequent
pancreas retransplantation. Arch Surg 1996;131:1054-60. Bragg LE, Thompson
JS, Burnett DA, Hodgson PE, Rikkers LF: Increased incidence of pancreas-related
complications in patients with postoperative pancreatitis. Am J Surg
1985;150:694-7. Büsing M, Hopt UT,
Quacken M, Becker HD, Morgenroth K: Morphological studies of graft pancreatitis following
pancreas transplantation. Br J Surg 1993;80:1170-3. Sugitani A, Gritsch
HA, Shapiro R, Bonham CA, Egidi MF, Corry RJ: Surgical complications in 123
consecutive pancreas transplant recipients: Comparison of bladder and enteric
drainage. Transplant Proc 1998;30:293-4. Troppmann C,
Gruessner AC, Benedetti E et al:Vascular graft thrombosis after pancreatic
transplantation: univariate and multivariate operative and nonoperative risk
factor analysis. J Am Coll Surg 1996;182:285-316. Wright FH, Wright C,
Ames SA, Smith JL, Corry RJ: Pancreatic allograft thrombosis: Donor and
retrieval factors and early postperfusion graft function. Transplant Proc
1990;22:439-41. Reddy KS, Stratta
RJ, Shokouh-Amiri MH, Alloway R, Egidi MF, Gaber AO: Surgical complications
after pancreas transplantation with portal-enteric drainage. J Am Col Surg
1999;189:305-13. Grewal HP, Garland
L, Novak K, Gaber L, Tolley EA, Gaber AO: Risk factors for postimplantation
pancreatitis and pancreatic thrombosis in pancreas transplant recipients.
Transplantation 1993;56:609-12. Tollemar J, Tydén G,
Brattström C, Groth CG: Anticoagulation
therapy for prevention of pancreatic graft thrombosis: Benefits and risks.
Transplant Proc 1988;3:479-80. Sasaki T, Pirsch JD,
Ploeg RJ et al: Effects of DR mismatch
on long-term graft survival in simultaneous kidney-pancreas transplantation.
Transplant Proc 1993;25:237-8. Drachenberg CB,
Papadimitriou JC, Klassen DK et al.: Evaluation of pancreas transplant needle biopsy: reproducibility and
revision of histologic grading system. Transplantation 1997;63:1579-86. Drachenberg CB,
Papadimitriou JC, Klassen DK et al: Chronic pancreas allograft rejection:
morphologic evidence of progression in needle biopsies and proposal of a
grading scheme. Transplant Proc 1999;31:614. Drachenberg CB,
Abruzzo LV, Klassen DK et al: Epstein-Barr virus-related post-transplantation
lymphoproliferative disorder in pancreas allografts: histological differential
diagnosis from acute allograft rejection. Hum Pathol 1998;29:569-77. Grekas D, Alivanis
P, Derveniotis F, Papoulidou N, Kaklamanis N, Tourkantonis A: Influence of
donor data on graft function after cadaveric renal transplantation. Transplant
Proc 1996;28:2957-8. Douzdjian V,
Gugliuzza KG, Fish JC: Multivariate analysis of donor risk factors for pancreas
allograft failure after simultaneous pancreas-kidney transplantation. Surgery
1995;118:73-81. Odorico JS, Heisey
DM, Voss BJ et al.:Donor factors affecting outcome after pancreas
transplantation. Transplant Proc 1998;30:276-7. Tso PL, Elkhammas
EA, Henry ML et al.: Risk factors of long-term survivals in combined
kidney/pancreas transplantation: A multivariate analysis of 259 recipients.
Transplant Proc 1995;6:3104. Hawthorne WJ,
Griffin AD, Lau H et al.: Experimental hyperacute rejection in pancreas
allotransplants. Transplantation 1996;62:324-9. Hesse UJ and
Sutherland DE: Influence of serum amylase and plasma glucose levels in pancreas
cadaver donors on graft functioning recipients. Diabetes 1989;38:1-3. 
Please mail comments, corrections or suggestions to the TPIS administration at the UPMC.
If you have questions, please email TPIS Administration. |
|