|
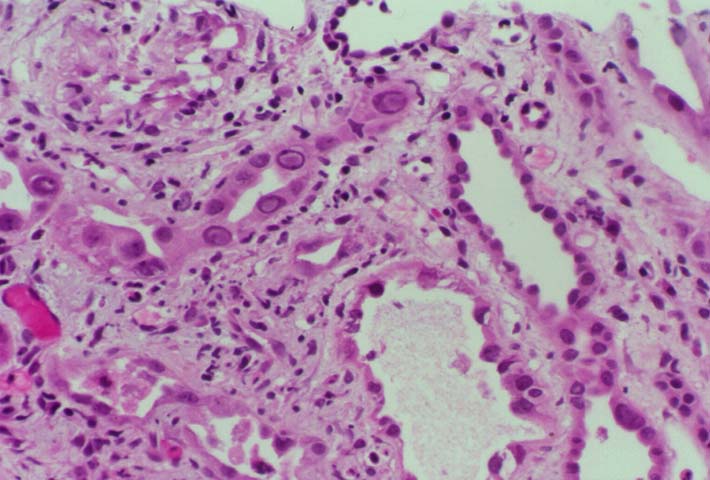
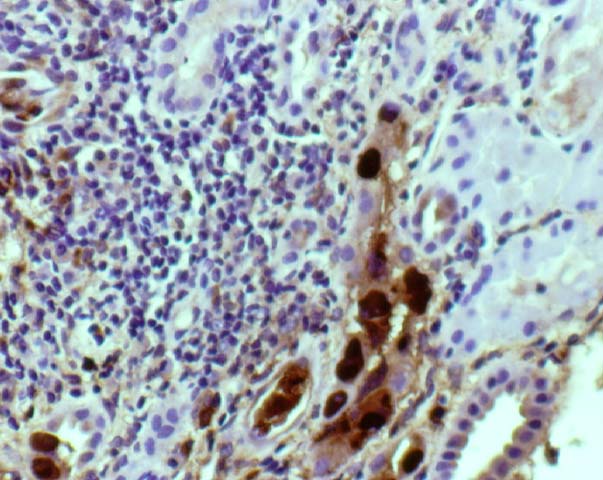
Background and Clinical Presentation The polyoma virus infections are acquired early in childhood, and 60-80% of adults in the U.S. test seropositive for these viruses. The majority of infections are subclinical and lead to viral latency within the kidney. Reactivation occurs in transplant recipients as a result of immunosuppressive therapy. Serologic evidence suggests that the donor kidney may act as the vehicle of transmission. BK or JC virus is shed in the urine of 10-60% of patients after renal transplantation, but clinically significant interstitial nephritis is infrequent. The typical presentation is a rise in the serum creatinine, which cannot be distinguished from rejection or drug toxicity on clinical grounds. A few cases show hydronephrosis on ultrasound examination at the time of allograft biopsy. Intravenous pyelography in these patients may demonstrate a ureteric stricture. Hemorrhagic cystitis is described after bone marrow transplantation. HistopathologyNeedle biopsy of the allograft kidney shows a mixed interstitial inflammatory infiltrate with focal tubular injury. The tubular epithelium shows marked anisonucleosis, nuclear atypia, and basophilic or amphophilic intranuclear inclusions. Tubulitis is frequently present, and satisfies the Banff criteria for acute rejection. In addition biopsies may show clusters of neutrophils in the tubular lumen suggestive of pyelonephritis. Pathologic Classification The American Society of Transplantation 2013 Classification categories/histologic patterns can be referenced in Am J Transplant 2013: 13: 179. In essence, Category A includes cases with viral cytopathic effect but little to no inflammation. Category B refers to biopsies with variable degrees of interstitial inflammation graded from B1 to B3. Category C designates presence of interstitial fibrosis and tubular atrophy affecting greater than 50% of the sampled tissue. By comparison, the Banff Working Group Classification of Definitive Polyomavirus Nephropathy (J Am Soc Nephrol 2018: 29: 680) relies primarily on viral load and interstitial fibrosis for prognostication and does not take into account the degree of inflammation (pvl = polyomavirus replication/load level):
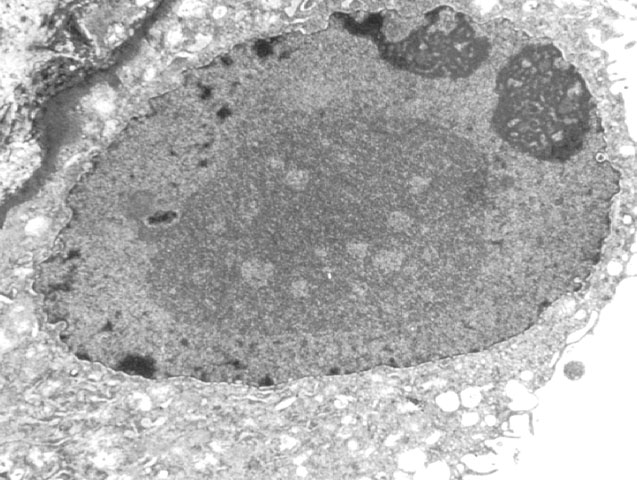
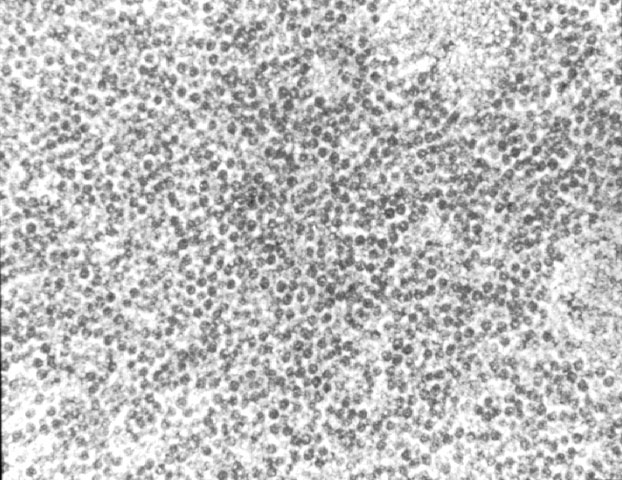
Acute tubular injury due to ischemic or immunologic injury can result in a florid regenerative response with extremely prominent nucleoli, which should not be confused with viral inclusions. Polyomavirus inclusions in biopsy tissue are intranuclear with a homogenous basophilic or amphophilic appearance, which is quite distinct from cytomegalovirus, Herpesvirus and adenovirus inclusions. Definite identification may be done by immunohistochemistry, in-situ hybridization, or PCR. Electron microscopy is also a useful tool in the differential diagnosis. Polyom virus particles measure 45-55 nm, while adenovirus measures 70-90nm. Herpes simplex and cytomegalovirus are enveloped virions with a size range of 120-160nm. In formalin fixed tissue viral particles can appear to be smaller than their expected size due to shrinkage artefact. Treatment No specific therapy is currently available for polyoma virus infections. Human immune globulins are administered by some clinicians, but their efficacy has not been established. Reduction in immunosuppression is helpful, and results in a decrease in the viral load in follow-up biopsies. Unfortunately, this strategy generally results in graft loss due to rejection. If ureteric stenosis is present, surgical intervention can potentially benefit cases with significant obstruction. However, the clinical response may be limited in cases with concurrent chronic allograft nephropathy.
References
Please mail comments, corrections or suggestions to the TPIS administration at the UPMC.
If you have more questions, you can always email TPIS Administration. |
|