|
Liver Allograft Chronic Rejection Study Reference |
 |
Organization:
- BIHB - Beth Israel Hospital, Boston
- BERL - Berlin, Germany
- BUMC - Baylor University
- HEID - Heidelberg, Germany
- KYOT - Kyoto, Japan
- MTSI - Mount Sinai Hospital, NYC
- UPMC - University of Pittsburgh
- RFHO - Royal Free Hospital, London, UK
- UBIR - University of Birmingham, UK
- UCHI - University of Chicago
- UCLA - University of California, Los Angeles
- VIEN - Vienna, Austria
PID: The Patient IDentification number to be used in combination with the CID and Organization code to create a unique 'key' for each record (range 001-999). Participating institutions should correlate with medical record numbers or other patient identifier(s) separately.
CID: The Case IDentification number to be used in combination with the PID and Organization code to create a unique 'key' for each record (range 01-99). Participating institutions should correlate with institutional accession numbers or other specimen identifier(s) separately.
Age: The chronological age of the patient specified in whole years (range 01-99).
Sex: The gender of the patient.
Date of Biopsy: The date that the biopsy was obtained in Day, Month and Year format.
Date of Transplant: The date that the allograft liver being evaluated was transplanted into the patient in Day, Month and Year format.
Transplant Interval: This value will be calculated from the dates provided for transplantation and biopsy.
Case Entry Criteria:
- CR fulfilled - All allografts in which chronic rejection was verified at the time of retransplantation, based on the criteria of obliterative arteriopathy affecting at least some of the peri-hilar arteries and/or the presence of bile duct loss involving at least 50% of ducts.
- CR not fulfilled - All allografts in which the cause of graft failure has no explanation other than rejection.
Original Disease [1]: The primary disease process that lead to the need for a liver allograft. Specify more detailed information in the 'Case Comments' text box.
Original Disease [2]: A second concurrent disease process that contributed to the need for a liver allograft, if present.
Original Disease [3]: A third concurrent disease process that contributed to the need for a liver allograft, if present.
Baseline Immunosuppression Regimen: The immunosuppressive regimen used immediately after the organ being evaluated was transplanted. Specify the specific regimen in 'Case Comments" if not present in the list of options (ie. 'Other').
Specimen Type: The type of specimen for the case being evaluated. Neeble Biopsy and Wedge Biopsy should not have information filled out for the "Hilum" section of the form, while Failed Allograft and Autopsy require the "Hilum" section to be complete.
TBil (mg/dl): The serologic value obtained in nearest chronological proximity to the liver specimen removal. Specify blood draw date (DD-MMM-YY) if > 1 week before or after liver specimen removal.
ALT (IU/L): The serologic value obtained in nearest chronological proximity to the liver specimen removal. Specify blood draw date (DD-MMM-YY) if > 1 week before or after liver specimen removal.
AST (IU/L): The serologic value obtained in nearest chronological proximity to the liver specimen removal. Specify blood draw date (DD-MMM-YY) if > 1 week before or after liver specimen removal.
ALP (IU/L): The serologic value obtained in nearest chronological proximity to the liver specimen removal. Specify blood draw date (DD-MMM-YY) if > 1 week before or after liver specimen removal.
GGTP (IU/L): The serologic value obtained in nearest chronological proximity to the liver specimen removal. Specify blood draw date (DD-MMM-YY) if > 1 week before or after liver specimen removal.
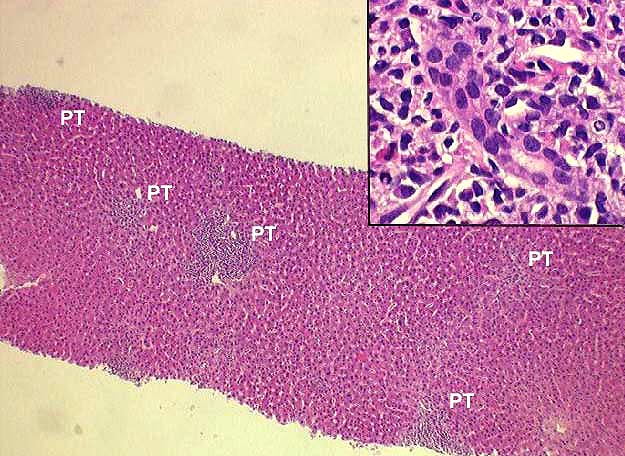
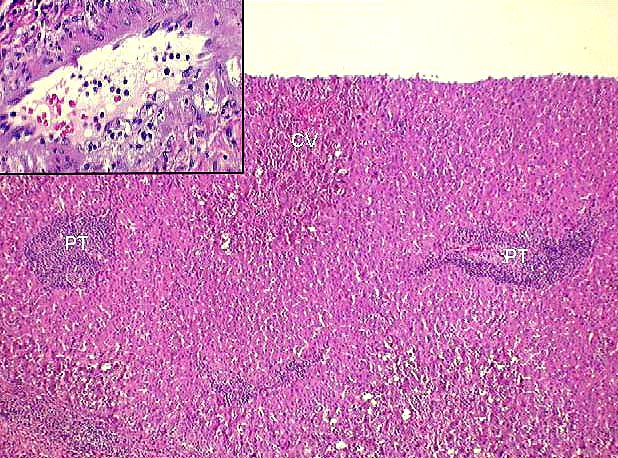
Portal Tracts
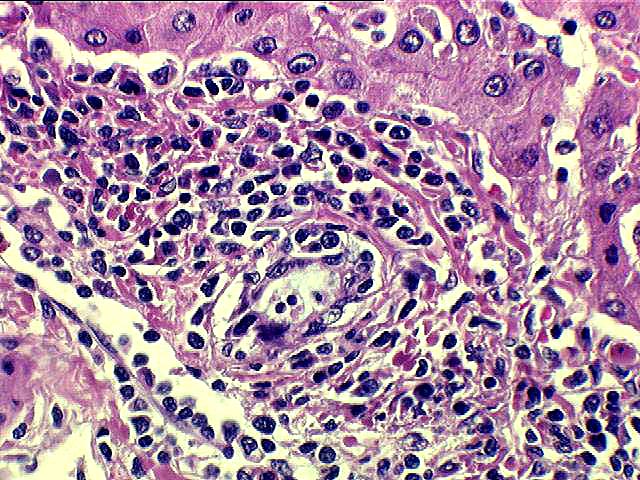
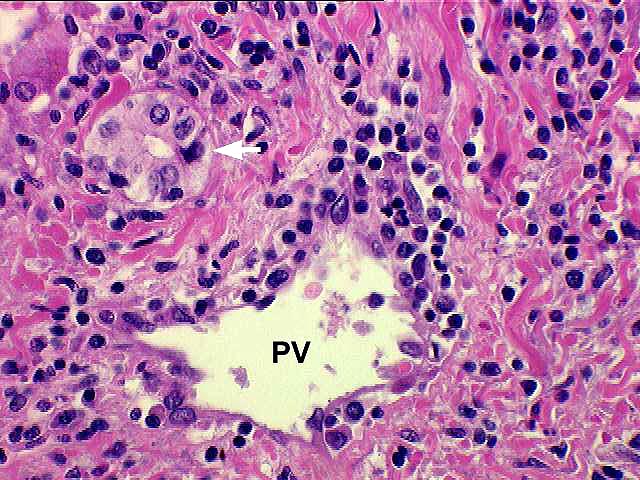
Inflammatory Severity: Graded (range 0-3) as per the Banff Schema ('97). Examples with discussion of these grades are available at the TPIS Liver Tx Topics reference resource:
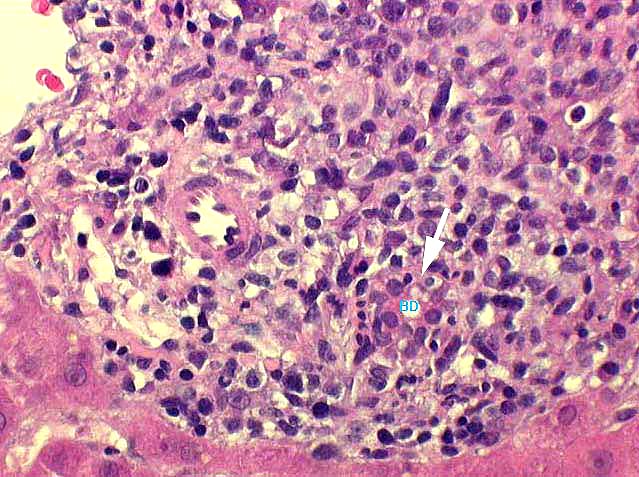
Bile Duct Inflammation/Damage: Graded (range 0-3) as per the Banff Schema ('97). Examples with discussion of these grades are available at the TPIS Liver Tx Topics reference resource:
Portal Vein Endotheliitis: Graded (range 0-3) as per the Banff Schema ('97). Examples with discussion of these grades are available at the TPIS Liver Tx Topics reference resource:
Total # Portal Tracts (count 50 if failed allograft): The total number of portal tracts present in the liver allograft specimen (count up to 50 if 'Specimen Type' = Failed Allograft or Autopsy).
# Portal Tracts without Bile Ducts: The total number of portal tracts without bile ducts. Should not exceed 'Total # Portal Tracts'.
# Portal Tracts without Arterial Branches: The total number of portal tracts without arterial branches. Should not exceed 'Total # Portal Tracts'.
Dysplastic Ducts: The relative quantity of dysplastic ducts.
Interface Activity: Range 0-3 (0=none present).
Fibrosis: Range 0-4 (0=none present).
 |
 |
 |
 |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
Arterial
Intimal Inflammation: Range 0-3 (0=none present).
Foam cells: Range 0-3 (0=none present).
Fibrosis: Range 0-4 (0=none present).
 |
 |
 |
 |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
Lobule / Parenchyma
Disarray: Range 0-3 (0=none present).
Overall Inflammation: Range 0-3 (0=none present).
Spotty Necrosis: Range 0-3 (0=none present).
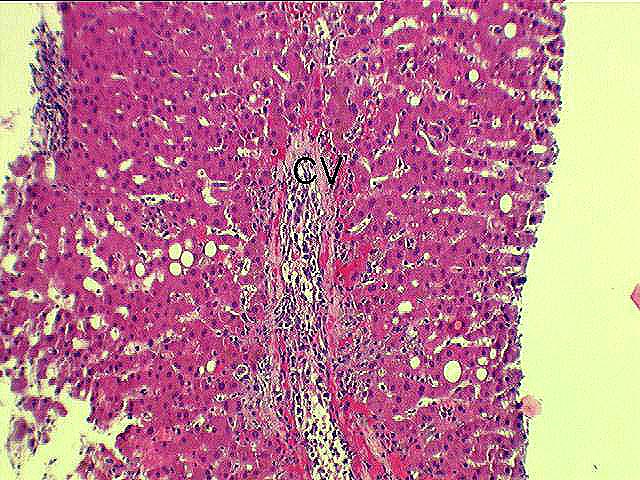
Perivenular
Inflammation: Range 0-4 (0=none present).
 |
 |
 |
 |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
Necrosis: Range 0-3 (0=none present).
Ballooning: Select if hepatocellular ballooning is identified in the specimen.
Congestion / Hemorrhage: Select if congestion and/or perivenular hemorrhage are present in the specimen.
Central Vein Endotheliitis: Range 0-3 (0=none present).
Foam Cell Clusters: Select if foam cell clusters are present in the specimen.
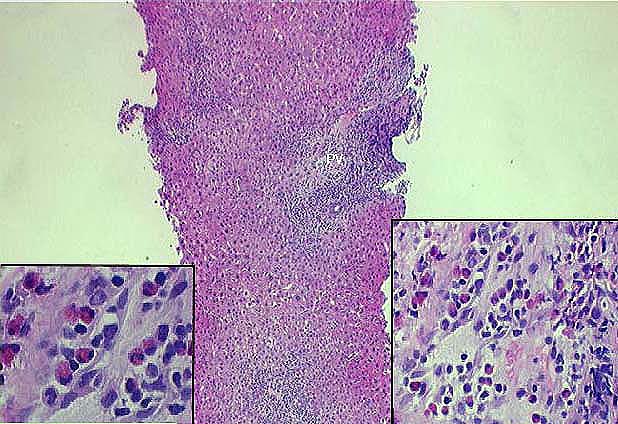
Cholestasis
Severity: Range 0-3 (0=none present).
Location: Specify predominant localization of the cholestsis if present. If Other is selected specify predominant location in 'Case Comments' text area.
CMV
PCR (Polymerase Chain Reaction): Select if CMV infection was demonstrated by PCR positivity in the allograft specimen.
In Situ Hybridization (ISH): Select if CMV infection was demonstrated by ISH positivity in the allograft specimen.
Microabscesses: Select if CMV infection was demonstrated by identifying microabscesses in the allograft specimen.
Inclusion Bodies: Select if CMV infection was demonstrated by identifying diagnostic intranuclear and/or cytoplasmic inclusion bodies in the allograft specimen.
Hilum (Failed Allografts and Autopsy Livers Only)
Liver Weight (gm): The weight of the removed allograft organ in grams.
Arteriopathy:
Large Bile Duct Inflammation:
Arterial intima
Foam Cells:
Myofibroblast:
Inflammation:
Media
Foam Cells:
Inflammation:
Adventitia
Foam Cells:
Fibrosis:
Inflammation:
Allograft Diagnosis
Allograft Diagnosis [1]:
Specific Diagnosis [1]:
Allograft Diagnosis [2]:
Specific Diagnosis [2]:
Case Comments:
Submit Information
Search Information
Delete Information
Please mail comments, corrections or suggestions to
TPIS administration. .