Antibody Mediated Rejection
Clinical presentation
Antibody mediated rejection of the kidney generally occurs hours to days after transplantation (hyperacute rejection), but may be delayed by several weeks if sensitization to donor antigens has occured in the remote past (delayed accelerated acute rejection). In the worst case scenario it begins immediately after perfusion of the allograft is established. The transplanted kidney becomes grossly mottled and cyanotic. The capsule bulges out due to marked edema and rupture of the graft may occur. The transplanted kidney never functions, and the patient returns to dialysis. Delayed cases of antibody mediated rejection present as an acute rise in the serum creatinine with or without graft tenderness.
Pathology
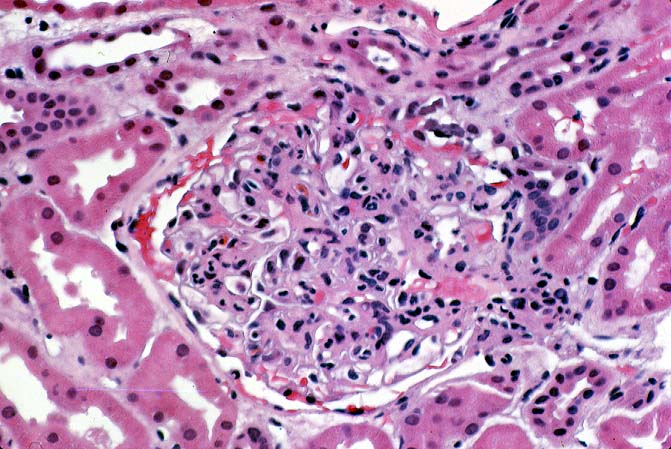
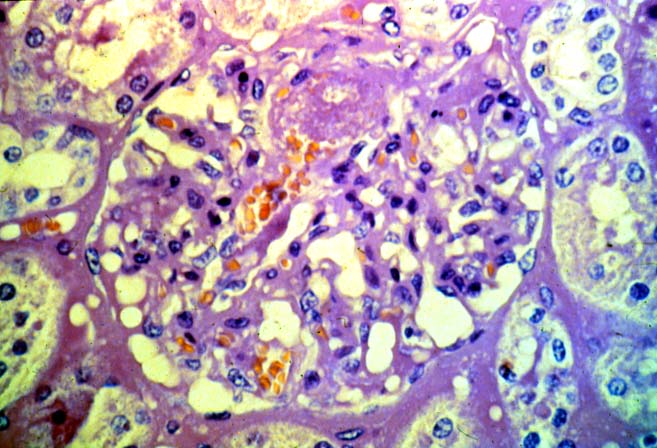
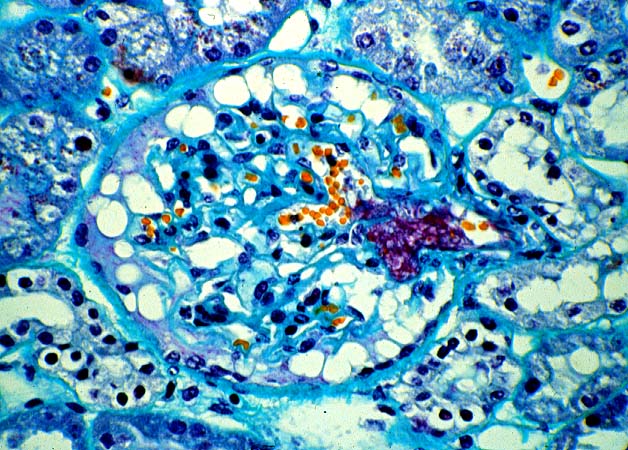
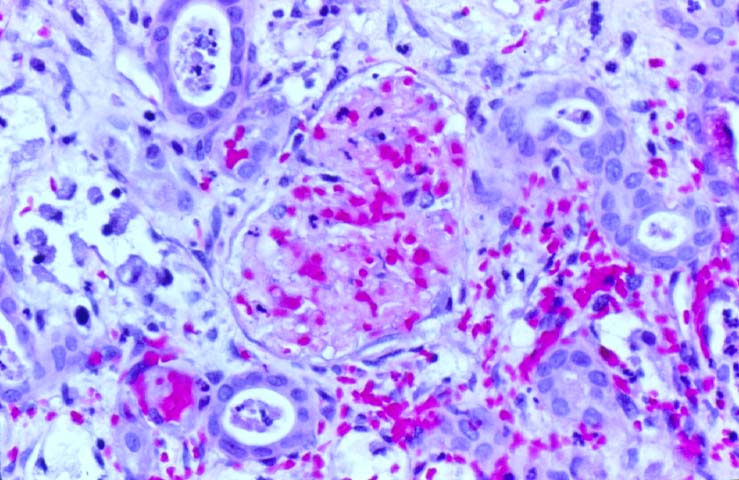
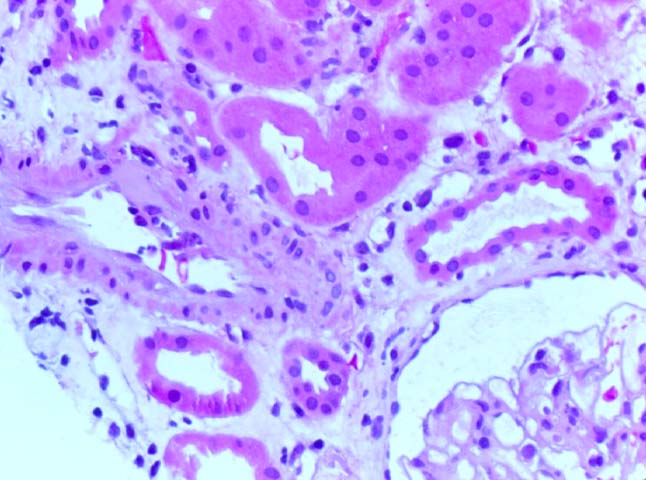
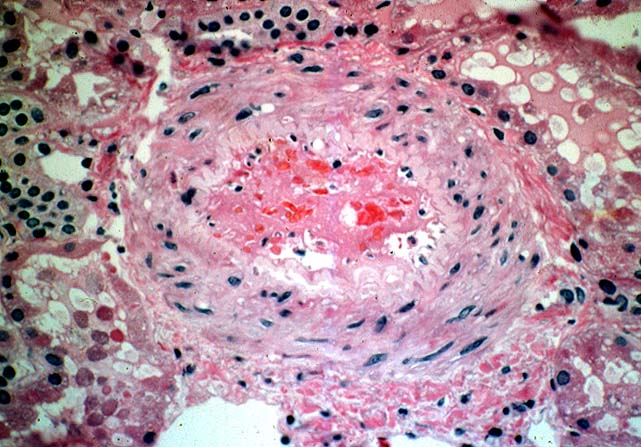
Histologically, the fully developed cases are characterized by widespread glomerular capillary thrombosis and necrosis. Frequent areas of interstitial hemorrhage are noted. In early cases, the changes may be subtle and limited to endothelial reactivity with associated sparse polymorphonuclear infiltrates. A diffuse margination of neutrophils is seen throughout the peritubular and glomerular capillaries. The presence of five or more neutrophils present in any glomerulus is distinctly abnormal.
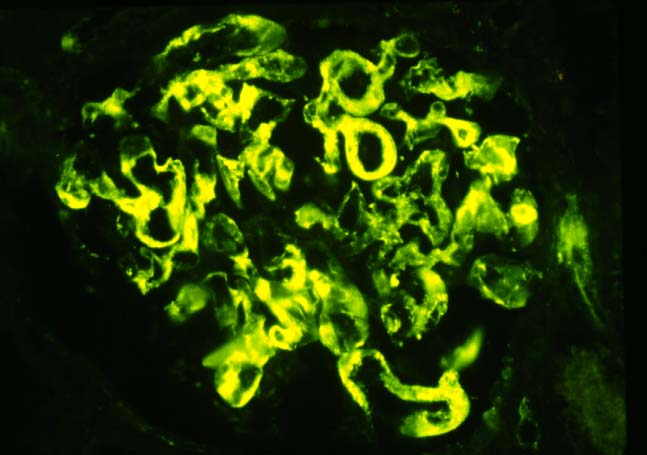
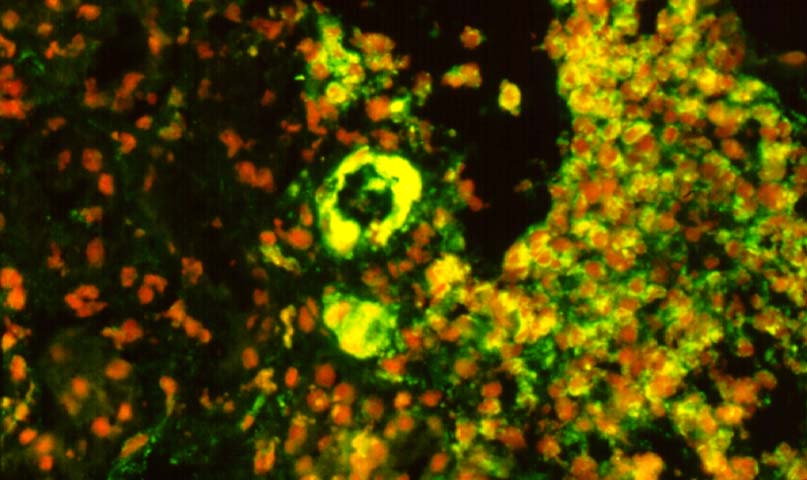
Immunofluorescence examination is a very helpful corroborative technique in establishing the diagnosis: immunoglobulin and complement deposits can be seen in the glomeruli, peritubular capillaries, and interlobular arteries. However several potential pitfalls need to be remembered in the evaluation of such deposits. IgG present in serum permeating the interstitial compartment can give an annoying background in the tissue being examined. IgM and C3 staining is often present non- specifically in vessels affected by arteriosclerosis or hypertensive vasculopathy. Indeed IgG, IgM and C3 deposits within arterioles have been found in baseline kidney biopsies from healthy living related donors. However, these non-specific immune deposits are said to be lumpy and irregular, whereas in acute vascular rejection, linear deposits outlining individual cells are typical. Finally a negative immunofluorescence does not exclude vascular rejection because the immunoglobulin and complement deposits can undergo degradation. In cases where immunofluorescent staining is difficult to interpret one can attempt elution of antibodies from the graft tissue and show the specifity of these antibodies to specific donor antigens.
Differential diagnosis
Histopathological changes mimicking acute vascular rejection can be seen in a variety of clinical settings. In renal allografts exposed to prolonged cold ischemia glomerular capillaries may lose their endothelial lining. Secondary thrombosis can then occur, but is usually bland in its appearance and not associated with the subendothelial inflammatory infiltrates characteristic of antibody mediated rejection. Cyclosporine and tacrolimus sometimes damage the vascular endothelium causing a thrombotic microangiopathy which may be difficult to distinguish from antibody mediated rejection. Severe hypertension can cause fibrinoid necrosis of the small arteries and thrombosis of the glomerular capillary loops. In patients undergoing transplantation for idiopathic hemolytic uremic syndrome, Henoch-Schonlein purpura or thrombotic thrombocytopenic purpura, recurrence of the original disease in the graft causes fibrin thrombi in the vessels as well as necrotizing glomerular lesions. Finally, systemic disseminated intravascular coagulation may be associated with similar histopathological changes. In all these situations other systemic manifestations of the underlying disorder are present and help in the differential diagnosis.
Pathophysiology
The pathogenesis of antibody mediated rejection occurring within the first few days of transplantation is believed to be due to preformed antibodies reactive against donor antigens. These antibodies develop during a prior exposure of the recipient to donor derived antigens in connection with a blood transfusion, pregnancy or abortion. If the titer of these antibodies has fallen off, a few days or even weeks may elapse before an amnestic immune response can develop. Antibody mediated rejection in such cases may be somewhat delayed and less severe. It is also possible for antibody mediated rejection to occur in unsensitized patients. In such cases, a primary or denovo cell- mediated immune response may be the primary pathogenetic mechanism, and such episodes of antibody mediated rejection are potentially reversible by treatment with anti-T cell drugs, such as cyclosporine, tacrolimus or anti-lymphocyte antibodies.
HLA and endothelium associated donor antigens are the usual target for antibody mediated rejection. Immune reactions mediated by antibodies to the ABO blood group system have now become uncommon and are usually the result of technical errors. In the past, deliberate attempts were made to breach the ABO blood group barrier, in instances where living related donors were HLA identical but ABO incompatible. The incidence of hyperacute acute rejection in these cases was unacceptably high in spite of aggressive immunosuppression. Somewhat better results were obtained when kidneys from blood group A2 donors were transplanted into blood group O recipients. This was likely related to low titers of anti A2 antibodies in the recipient and to the occurrence of ABO-non secretors in the human population. Nonetheless, the long-term survival of these individuals was not comparable to that which can be presently obtained with ABO compatible donor-recipient combinations.
The severity of antibody mediated rejection can vary from case to case. In fact, not all transplant recipients with donor specific antibodies demonstrable pre-operatively actually go on to develop hyperacute rejection. This variation may have to do with the actual titers of antibodies present. The binding affinity of these antibodies might also vary from person to person, as could the intensity of expression of HLA and other donor specific antigens within the allograft. Other possible moderating influences include the participation of CD8 antigen positive T-suppressor cells, anti-idiotypic antibodies and the type/intensity of the immunosuppressive agents used.
Prophylaxis and treatment
Antibody mediated rejection is frequently associated with graft loss. However early and less severely affected cases can be salvaged by increasing the dosage of cyclosporine/tacrolimus and administration of antilymphocyte antibodies. In highly presensitized cases with high levels of circulating antibodies plasmapheresis can be tried if the patient is hemodynamically stable. In general, treatment of full blown antibody mediated rejection is disappointing, and it is better to minimize the possibility of its occurrence by performing a donor specific cross match and a panel reactive antibody (PRA) test on the recipient serum prior to transplantation. In most transplant centers donor specific cross-matches are still performed using peripheral blood lymphocytes. Such tests do not detect anti- endothelial antibodies, and this accounts for at least some cases of the antibody mediated rejection reported in lymphocyte crossmatch negative patients.
References
|
|
|
|