
Renal Electronmicrographs
Figure 1. Transplant glomerulitis
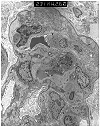
Electron micrograph from a renal allograft biopsy, which showed borderline acute rejection and mild chronic allograft nephropathy at light microscopy. The illustration provided shows glomerular capillary loops containing several mononuclear, and occasional polymorphonuclear cells. The endothelial cells show swelling and accentuation of the cytoplasmic processes. The endothelial zone contains granular proteinaceous material. This combination of intracapillary mononuclear cells and endothelial injury is a lesion referred to transplant glomerulitis in the Banff Schema. In our experience, this change is commonly observed in conjunction with acute rejection. Some cases are reportedly associated with drug toxicity and CMV infection. The absence of immune complex deposits differentiates this lesion from endocapillary glomerulonephritis.
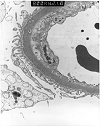
Figure 2. Transplant glomerulitis
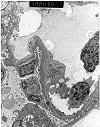
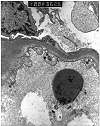
Electron micrographs from an allograft kidney showing the lesion referred to as "transplant glomerulitis" in the Banff Schema. The glomerular capillaries contain mononuclear cells associated with endothelial injury in the form of cytoplasmic bleb formation and intracytoplasmic tubulo-reticular inclusions.
BACK TO TOP
Figure 3. Chronic transplant glomerulopathy
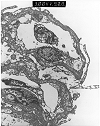
Electron micrograph prepared from a renal allograft biopsy performed several years after transplantation for chronic glomerulonephritis. Light microscopy showed changes consistent with moderate chronic allograft nephropathy, but it was not possible to exclude recurrent disease. Glutaraldehyde fixed tissue was not available, so ultrastructural evaluation was attempted on paraffin embedded tissue. The micrograph provided for review shows relatively poor preservation of morphology. Nonetheless, it demonstrates marked thickening and duplication of the glomerular basement membranes with incorporation of cellular debris. The absence of immune complex deposits (which usually survive well even in paraffin embedded tissue) helps to make a diagnosis of chronic transplant glomerulopathy in this case.
BACK TO TOP
Figure 4. Chronic transplant glomerulopathy
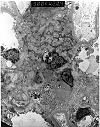
Two electron micrographs showing changes of chronic transplant glomerulopathy. There is increased mesangial matrix and cellularity, with several mesangial cells showing prominent cytoplasmic processes. The subendothelial zone is expanded by the presence of a granular electron lucent material with numerous membranous and filamentous profiles. Tubuloreticular inclusions (TRI's) are seen within the endothelial cytoplasm. TRI's are classically described in systemic lupus erythematosus and viral infections, but may also be found in renal allograft biopsies without implying the presence of either of these disease.
BACK TO TOP
Figure 5. Chronic ischemic glomerulopathy
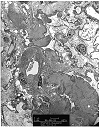
Electron micrograph from a renal allograft biopsy, which showed moderate to severe chronic allograft nephropathy by light microscopy. The photomicrograph provided shows marked thickening and wrinkling of the glomerular basement membranes. Several capillary loops are expanded by a variably electron dense proteinaceous insudate containing scattered vacuoles. These
changes are indicative of ischemic glomerulopathy secondary to arteriosclerosis.
BACK TO TOP
Figure 6. Recurrent membranous glomerulonephritis
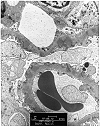
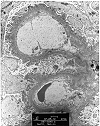
Needle biopsy of an allograft kidney from a patient with heavy proteinuria, following transplantation for membranous glomerulonephritis. The H & E stain showed 2 glomeruli with minimal abnormality. Focal thickening of the glomerular basement membranes was seen on PAS. The silver stain showed no spikes, but revealed a soap-bubble change within the glomerular basement membranes. On electron microscopy, the pale staining bubbly areas correspond to subepithelial electron dense deposits. These findings are consistent with recurrent membranous glomerulonephritis.
BACK TO TOP
Figure 7. Recurrent membranous glomerulonephritis
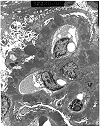
Electron micrograph of a renal allograft biopsy performed four years after transplantation. Ultrastructural examination (performed on paraffin embedded tissue) shows massive "hump" shaped subepithelial electron dense deposits associated with duplication and spike formation in the glomerular basement membranes. These changes reflect recurrent membranous glomerulonephritis, stage 3 in the allograft kidney. While "hump" shaped subepithelial deposits are most characteristic of post-streptococcal glomerulonephritis, they may also be seen in other glomerular diseases.
BACK TO TOP
Figure 8. Recurrent IgA nephropathy
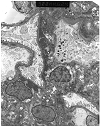
Electron micrograph prepared from a renal allograft biopsy showing changes consistent with chronic rejection on light microscopy. Ultrastructural examination shows paramesangial, mesangial and subendothelial deposits consistent with recurrent IgA nephropathy. A definitive diagnosis would require documentation of IgA dominant or codominant immunoglobulin deposits within the glomeruli using immunofluorescence techniques. Recurrent IgA nephropathy is common, but does
not usually cause graft loss by itself.
BACK TO TOP
Figure 9. Recurrent systemic lupus erythematosus
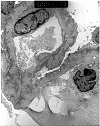
Electron micrograph prepared from a renal allograft biopsy performed three years after transplantation for systemic lupus erythematous. Light microscopy showed changes, which were interpreted as chronic transplant glomerulopathy. The photomicrograph provided shows suboptimal morphological preservation, since ultrastructural examination was performed on paraffin embedded tissue. Nonetheless, it is possible to appreciate numerous subepithelial deposits, consistent with recurrent systemic lupus erythematosus (SLE). A definitive diagnosis would require clinical exclusion of other causes of membranous glomerulonephritis. Recurrence of SLE in the allograft kidney is a relatively uncommon clinical event. The risk of recurrence can be further reduced by delaying transplantation till the disease has become clinically inactive.
BACK TO TOP
Figure 10. Recurrent focal segmental glomerulosclerosis
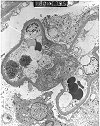
Renal allograft electron micrograph prepared from a patient who developed 11 gram/24 hr proteinuria 5 months after transplantation. The glomerular basement membranes show extensive fusion of the podocyte foot processes. The podocyte cytoplasm exhibits prominent microvillous transformation. In the appropriate clinical setting, these findings can be used to make a diagnosis of recurrent focal segmental sclerosis, even if segmental sclerosis lesions are not actually seen in biopsy material due to sampling considerations.
BACK TO TOP
Figure 11. De novo membranous glomerulonephritis
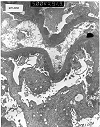
Electron micrograph from the allograft kidney of a patient reportedly transplanted for membranoproliferative glomerulonephritis Type 1. Assuming that the native glomerular disease was correctly classified, the presence of subepithelial deposits in the present specimen is indicative of denovo membranous glomerulonephritis. In some such cases, an underlying cause such as hepatitis, captopril therapy, or past administration of anti-horse thymocyte globulin can be identified. Other cases appear to be the result of immunologic injury directed against allograft antigens.
BACK TO TOP
Figure 12. De novo membranous glomerulonephritis
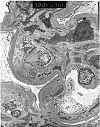
Electron micrograph prepared from a renal allograft biopsy which showed changes consistent with chronic rejection on routine H&E staining. A Masson's silver trichrome stain showed glomerular basement membranes with marked reduplication and bubbly change. This prompted further evaluation of the case by immunofluorescence and electron microscopy. Immunofluorescence showed immunoglobulin and complement deposits within the glomerular basement membranes, including the presence of properdin, reflecting activation of the alternate pathway of complement activation. The electron micrograph provided shows mesangial interposition, i.e. the presence of mesangial cell processes insinuating between the subendothelial and subepithelial aspects of the glomerular basement membranes. In addition, numerous immune complex deposits are seen in a subendothelial location. The native renal disease in this patient was chronic pyelonephritis. Hence, the ultrastructural findings were interpreted as being indicative of a de novo membranoproliferative glomerulonephritis, type I.
BACK TO TOP
Figure 13. De novo mesangioproliferative glomerulonephritis
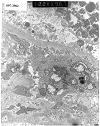
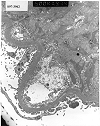
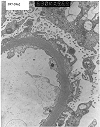
Electron micrographs from a renal allograft biopsy prompted by proteinuria developing 2 years after transplantation for diabetes. Expanded mesangial matrix, increased mesangial cell processes and subendothelial translucent debris are consistent with chronic transplant glomerulopathy. Print #967 shows small paramesangial electron dense deposits. The immunofluorescence staining pattern (IgM 3+, IgA 2+, C3 3+, C4 3+, fibrinogen 2+) was consistent with immune complex deposition, making it difficult to exclude de novo mesangioproliferative glomerulonephritis. Examination of serial biopsies is helpful in such cases. Minimal immune complex deposits, which do not progress with time, are felt to be within the spectrum of changes described in chronic transplant glomerulopathy. Another relatively infrequent finding in this case is the accumulation of cell debris on the epithelial aspect of the glomerular basement membrane.
BACK TO TOP
Figure 14. Denovo cryoglobulinemic glomerulopathy

Electron micrograph prepared from renal allograft biopsy performed six years after transplantation for an uncharacterized glomerular disease. There is prominent deposition of a finely granular and tubulo-filamentous material, which stained for IgG, IgM, kappa & lambda light chains, C1q, C3, C4, Albumin and Alpha 2 macroglobulin. The differential diagnosis includes amyloidosis, light chain disease, fibrillary glomerulonephritis, and cryoglobulinemic glomerulopathy.This patient had chronic liver disease, which is known to be associated with circulating cryoglobulins. Congo red staining for amyloid was negative.
BACK TO TOP
Figure 15. Tacrolimus toxicity
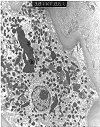
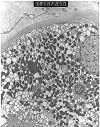
Tubular cytoplasmic vacuolization and giant mitochondria are used to suggest a diagnosis of cyclosporine and tacrolimus toxicity in renal allograft biopsies. The occurrence of these lesions in the provided electron micrographs prepared from a native kidney biopsy illustrates their non-specific nature. The diagnosis of drug toxicity is one of exclusion and should be made only after appropriate clinico-pathologic correlation.
BACK TO TOP
Figure 16. Tacrolimus toxicity
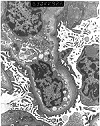
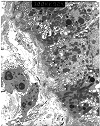
Electron micrographs from a renal transplant recipient with tacrolimus associated isometric tubular vacuolization at light microscopy. Ultrastructural examination was performed, because the clinical history of proteinuria made it desirable to exclude glomerulonephritis. The glomeruli show thickened capillary basement membranes with subendothelial deposition of finely granular electron-lucent material, consistent with early transplant glomerulopathy. No immune complex deposits are seen to support a diagnosis of glomerulonephritis. The ultrastructural counterpart of isometric vacuolization is found to be a dilatation of the endoplasmic reticulum with increased lysosomes.
BACK TO TOP
Figure 17. BK virus infection

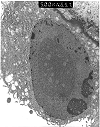
Electron micrographs from a renal allograft biopsy showing BK virus inclusions on light microscopy. The tubular nucleus depicted in the lower power micrograph shows margination of the chromatin, with the central part of the nucleus filled by a large mass of spherical particles arranged in crystalline arrays. At higher power examination the particles measured 30 nm, consistent with BK virus infection, if allowance is made for shrinkage following formalin fixation (theoretical virion diameter=45nm). The morphologic preservation of the tissue is suboptimal, since only paraffin embedded tissue was available for examination. Nonetheless, electron microscopy was clinically useful in substantiating the diagnosis. Subsequently, BK virus specific antibodies and probes were obtained, and the diagnosis was further confirmed by immunohistochemistry and in-situ hybridization.
BACK TO TOP
























