|
Introduction
Organ transplantation has become a therapeutic modality to treat
patients with end-stage disease. While many types of organs have
been successfully transplanted, the histocompatibility barrier
between recipient and donor remains a problem in that it will
activate immune responses leading to graft rejection. Although
immunosuppressive drugs such as tacrolimus (FK-506) and cyclosporine
will reduce rejection, the successful management of the transplant
patient requires an understanding of the Major Histocompatibility
Complex (MHC) also referred to in humans as the HLA (or Human
Leukocyte Antigen) system. This overview addresses some biological
aspects of HLA in relation to issues of histocompatibility testing
in transplantation.
Role of HLA in Transplant Immunity
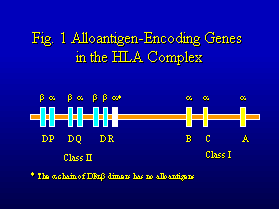
These genes have been classified into major categories. HLA-A,
HLA-B and HLA-C encode for Class I molecules consisting of a 45kD
glycopeptide chain complexed to a 12kD b2-microglobulin
chain encoded by a nonpolymorphic gene on chromosome 15 (Figure
2). The genes in the HLA-DR, HLA-DQ and HLA-DP regions encode
for Class II molecules consisting of a ~30kD a-chain
and a ~28kD b-chain. These HLA class
I and class II alloantigens can induce transplant immunity at
both humoral (antibody) and cellular (T lymphocyte) immune levels.
The human MHC contains many other Class I and Class II genes (e.g.
HLA-G and HLA-DM, respectively) whose products do not seem important
as transplantation antigens. A third set of so-called Class III
genes controls a heterogeneous group of proteins that include
Complement components C2, C4 and Factor B, Tumor Necrosis Factor,
21-Hydroxylase and Heat Shock Protein-70.
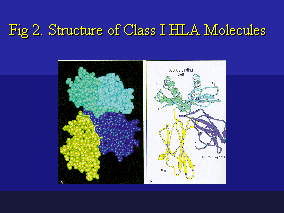 Structurally, HLA molecules are cell surface-bound glycoproteins that contain four immuno-globulin-like domains. The two external domains form a peptide-binding groove consisting of two parallel a-helix amino acid chains on an eight b-pleated polypeptide sheet (Figure 4). The peptide-binding grooves of Class I and Class II molecules are structurally analogous. Their primary role is to bind small antigenic peptides for presentation to the T-Cell Receptor (TCR) which then may lead to specific T-Cell activation. Many antigenic peptides are generated or "processed" by so-called antigen-presenting cells (APC) and they can vary in length: from 8-9 amino acid residues bound to Class I molecules to 10-25 residues bound to Class II molecules.
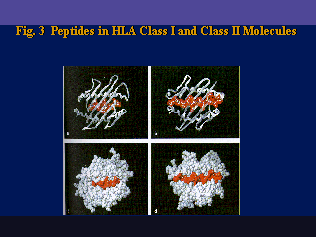
The considerable polymorphism of HLA is well-known. By serology
for instance, at least 25 alleles have been reported for HLA-A,
60 alleles for HLA-B and 18 alleles for HLA-DR. Molecular typing
has resulted in even much greater numbers of HLA alleles and it
is often very difficult to find HLA-matched unrelated donors for
transplant recipients. The polymorphism of HLA is reflected by
allelic substitutions of many amino acid residues in the polypeptide
chains, especially the external domains which contain the peptide-binding
site. This affects the spectrum of antigenic peptides presented
by the different allelic types of HLA molecules and the repertoire
of responding T-cells.
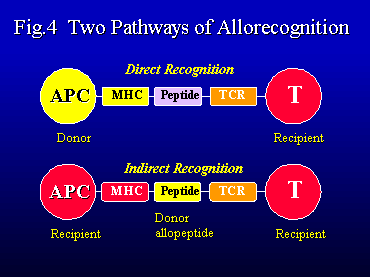
Functionally, HLA molecules play a crucial role in T-cell activation
by APC. This antigenic recognition depends on the interaction
between the antigenic peptide-binding HLA molecule and the T-Cell
Receptor (TCR), an immunoglobulin-like heterodimeric protein expressed
on T-lymphocytes. Two general pathways of T-cell alloactivation
have been recognized in transplant immunity (Figure 4). The direct
pathway refers to the alloreactive responses of recipient T-cells
to donor APC expressing incompatible HLA antigens. It provides
a powerful mechanism of T-cell alloactivation. In the indirect
pathway, allogeneic HLA antigens are taken up and processed by
recipient APC and presented in context with autologous HLA molecules
to recipient T-cells.
The transplanted organ represents a continuous source of HLA alloantigens
that can induce a rejection response at any time post-transplant.
Both alloactivation pathways are important in the generation of
donor-specific cell-mediated cytotoxicity and delayed-type hypersensitivity
(DTH)-like mechanisms of allograft rejection. Conversely a continuous
presence of donor MHC antigens is also needed for the maintenance
of allograft tolerance.
HLA is also involved in other cellular immune mechanisms that
affect transplant recipients. In Graft-Versus-Host (GVH) disease,
donor-derived immuno-competent lymphocytes react with HLA-incompatible
recipient cells and induce inflammatory responses in host tissues
such as the skin and gastrointestinal tract. This complication
is frequent after bone marrow transplantation, but may also affect
recipients of liver and other organ transplants and even blood
transfusions. GVH disease seems more likely in situations whereby
the donor is well matched for the patient but not the other way
around.
During infection, microbial antigens are processed by APC and
presented via HLA molecules to T-cells that elicit cytotoxic and
DTH-like inflammatory reactions in the allograft. The so-called
HLA-restricted mechanisms are more effective if the relevant HLA
antigens are shared between recipient T-cells and donor APC or
target cells. Recurrent autoimmune disease represents another
potential problem. Although end-stage organ failure due to autoimmune
disease can be successfully treated with transplantation, HLA-restricted
T-cell autoimmunity can be expected to persist in the patient.
This may promote recurrent disease especially, if the donor organ
shares the relevant HLA antigens with the recipient. Thus, HLA
compatibility can under certain conditions, promote certain non-alloimmune
mechanisms of allograft injury. The table below summarizes the
HLA-related cellular immune mechanisms that might affect organ
allograft outcome.
Direct and indirect HLA allorecognition mediate rejection and
conversely, GVH reactions if immunocompetent donor cells recognize
recipient incompatibilities. HLA-restricted immune responses to
microbial (viral) antigens and autoantigens can mediate non-rejection-related
inflammatory mechanisms. HLA matching can have a dualistic effect
on transplant outcome: on one hand, it reduces rejection but conversely,
it may promote other HLA-restricted mechanisms of allograft injury.
For most transplants, the HLA type of the donor can be expected
to have incompatibility for many HLA loci but compatibility for
some HLA loci. This would mean that both allospecific and self-HLA-restricted
immune mechanisms can occur simultaneously and may even synergize
in mediating inflammatory injury of the allograft..
Histocompatibility in Organ Transplantation
HLA matching clearly improves the survivals of transplanted kidneys,
hearts and lungs, but an HLA-based donor organ allocation has
been implemented only for kidney transplantation. This system
considers ABO and HLA compatibility of the donor and the results
of the lymphocytotoxicity crossmatch test between patient serum
and donor cells. Time constraints regarding the preservation of
donor hearts and lungs do not permit prospective HLA matching
for these organs. In some instances, crossmatching is done for
cardiothoracic transplantation especially if the patient is HLA
sensitized.
In kidney transplantation, current criteria for HLA matching consider
three loci: HLA-A, HLA-B and HLA-DR; each allele is defined as
a single antigen assigned by serological typing criteria, e.g.
HLA-A1, HLA-B44, HLA-DR7, etc. Each donor and recipient can type
for up to six different HLA antigens encoded by these loci and
HLA compatibility is usually assessed by the number of HLA mismatches
(or matches) of the donor. Many studies have shown a stepwise
decrease in graft survival of cadaver kidneys with increasing
numbers of HLA. The superior results with zero HLA-A,B,DR mismatches
have led to a system of mandatory sharing of such donor kidneys.
Nevertheless, a rather small proportion of recipients, especially
African-American patients, benefit from this system.
During recent years, alternative strategies for HLA matching have
been considered in kidney transplantation. They are referred to
as CREG (Cross-Reactive Group) matching, or "public"
epitope matching (the conventional HLA antigens are called "private"
epitopes) or, residue matching (determined from amino acid residue
sequence information of HLA antigens). All are based on the concept
that HLA molecules contain multiple antigenic determinants many
of them are shared and, that some are more important for matching
than others. CREG matching strategies are now being implemented
in kidney transplantation.
Histocompatibility testing for liver transplantation remains somewhat
of an enigma. Many investigators have noted that HLA compatibility
does not seem to benefit the overall group of liver transplant
recipients. In fact, several studies have shown lower survivals
of HLA-DR matched livers. HLA matching seems to has a dualistic
effect on liver transplant outcome: it reduces graft rejection
but it promotes other immune mechanisms of graft injury related
to viral infection (e.g. cytomegalovirus and hepatitis viruses)
and recurrent disease. Moreover, a liver allograft has a distinguished
feature of promoting a hematolymphoid chimeric state associated
with transplant tolerance but liver graft-derived immunocompetent
cells may also induce GVH disease. Donor-specific crossmatching
has limited relevance to liver transplantation because the liver
allograft is relatively resistant to humoral rejection . In some
sensitized patients, a liver allograft may even protect a subsequent
kidney transplant from hyperacute rejection.
Tissue Typing for Clinical Transplantation
The main purpose of tissue typing in transplantation is (1) to
assess donor-recipient compatibility for HLA and ABO and (2) to
analyze patient serum for antibodies that react with transplant
donor tissues. Most relevant is the crossmatch assay whereby patient
sera are tested for their reactivity with donor lymphocytes. This
is usually done by lymphocytotoxicity testing whereby donor lymphocytes
are first incubated with patient serum, then with rabbit complement
and lysis of lymphocytes is assessed by the uptake of an extravital
dye like trypan blue or eosin red. A positive crossmatch is a
contraindication for organ transplantation because of the risk
for hyperacute rejection and the higher incidence of vascular
rejection during the early post-transplant period. This applies
particularly for kidney and heart transplants whereas the liver
allograft is more resistant to antibody-mediated injury.
In kidney transplantation, several modifications of the crossmatch
assay have been used to increase its sensitivity including anti-human
globulin (AHG) augmentation, flow cytometry and enzyme-linked
immunoassays (ELISA) and B-cell crossmatches. Serum treatment
with dithiothreitol (DTT) is used to distinguish clinically irrelevant
IgM type antibodies.
Transplant candidates may become sensitized following a prior
transplant, blood transfusions and previous pregnancies. Serum
screening for alloreactive antibodies against a random cell panel
will provide an assessment of the degree of sensitization expressed
as the percentage Panel Reactive Antibody (PRA). The PRA can vary
between 0% (non-sensitized) to 80-100% indicating a high degree
of sensitization. Patients with high PRA values are less likely
to have crossmatch-negative donors. They must wait much longer
for a transplant and some may never receive a kidney.
Several techniques have been used to screen patient sera for alloreactive
antibodies. The complement-dependent lymphocytotoxicity technique
has two versions, one is a direct assay of patient antibodies
exert a lymphocytotoxic effect through complement-dependent mechanisms
of cell membrane lysis. Examples are the NIH (or "standard")
and the Amos modified techniques. The indirect technique utilizes
an extra step with a goat or rabbit anti-human IgG antibody-mediated
augmentation of complement-dependent lymphocytoxicity. This antiglobulin
(or AHG) modification is more sensitive than the direct lymphocytotoxicity
but is also technically more demanding. The source of lymphocytes
is an important consideration, several laboratories utilize B-cell
screening to detect HLA class II-specific antibodies.
Recently, additional serological methods have been developed that
do not utilize lymphocytotoxicity as an endpoint. One is based
on flow cytometric analysis of alloantibodies binding to panel
donor lymphocytes with different HLA types. Serum screening is
also now being done with an ELISA assays using solubilized HLA
antigens immobilized on a solid surface.
An important goal of any serum screening procedure is to gain
insight about the spectrum of HLA-specific antibodies, especially
in highly sensitized patients. This is based on the concept that
each HLA molecule has multiple antigenic determinants (epitopes)
which can be grouped as private (e.g. HLA-A1, HLA-B7, etc.) and
public determinants (sometimes referred to as crossreactive groups,
CREGs). A public determinant is an epitope shared by molecules
with different private specificities (e.g. A1+A3+A11, A2+A9+A28,
B5+15+35, B7+22+27+40, etc.). Most highly sensitized patients
show a persistence of the same pattern of antibody specificity
against one or a few public epitopes. A better understanding of
the antibody specificity improves the selection of transplant
donors with acceptable HLA mismatches.
Alloreactive T-lymphocytes are the primary mediators of cellular
rejection, and a considerable research effort has emphasized the
Mixed Leukocyte Culture (MLC) as an in vitro system the determine
T-cell alloreactivity between donor and recipient. These MLC assays
measure proliferative responses of alloactivated lymphocytes and
offer also opportunities to test for cell-mediated cytotoxicity,
cytokine production and the quantitation and functional characterization
of primed T-cells. Since these MLC procedures are technically
demanding and time-consuming, they are not routinely used for
prospective histocompatibility testing in a clinical service laboratory.
Considerable evidence has been obtained that matching for HLA
will reduce cellular rejection thereby promoting survival of kidney
and heart transplant patients. While the HLA system comprises
multiple class I and especially class II genes, most matching
strategies consider only three loci: HLA-A, HLA-B and HLA-DR.
Although it makes sense to find a perfect match for each transplant
patient, the reality of clinical practice dictates the selection
of less well-matched donors. As noted above, current strategies
are directed towards the identification of donors permissible
or acceptable HLA mismatches.
Conclusions
Because HLA plays such a dominant role in transplant immunity,
pre-transplant histocompatibility testing seems important for
organ transplantation. The table below summarizes the most commonly
used tissue typing procedures for clinical transplantation.. The
practical problem remains whether tissue typing can be applied
on a prospective basis for all types of transplants. Organ preservation
time remains an important limitation for an HLA-based tissue allocation
strategy especially hearts, lungs and intestines. Nevertheless,
histocompatibility testing yields relevant information for the
clinical management of any type of transplant recipient.
If you have more questions, you can always email TPIS Administration. |
|||||||||||||||||||||||||||||||