Introduction and Pathophysiology
The Epstein-Barr virus(EBV) has already infected a majority of the general population. After an initial infection which is often asymptomatic, it lies dormant in some epithelial cells and B lymphocytes. If the infected B lymphocytes are placed in tissue culture, the absence of T cell regulatory influences allows the B cells proliferate and become "immortalized", a process hastened by the presence of immunosuppressive drugs(1-7). Viral latency in vivo is maintained by T cell immune surveillance, which keeps viral replication and B cell proliferation in check. However, the potent immunosuppressive therapy needed to assure allograft acceptance depresses immune surveillance and predisposed the recipient to EBV activation or more serious manifestations of primary infections.
As with the other "opportunistic" viruses in the Herpes Virus group, the incidence of significant EBV-related disease is higher in liver allograft recipients who were seronegative before transplantation, but received an allograft from a seropositive donor(1-7). It is also more common in patients that receive heavy immunosuppression for treatment of rejection. In general, about 2% - 3% of adult recipients will develop persistent and/or recurrent disease. This can eventually result in the one of the most feared complication of EBV infection, which is development of a posttransplant lymphoproliferative disorder(1-7) or smooth muscle neoplasm(8, 9). PTLD refers to the emergence of oligoclonal and/or monoclonal B cell proliferations, that sometimes act like aggressive lymphomas(7, 10). A more detailed discussion of the pathophysiology of PTLD and the role of the Epstein-Barr virus is given in the relevant TPIS section on Neoplasia. The interested reader is also referred to several excellent reviews(7, 10-13).
Clinical Presentation
The systemic viral syndrome associated with EBV often resembles that seen with classical infectious mononucleosis: fever, lymphadenitis, pharyngitis, and jaundice(1-7) are the typical findings. Atypical signs and symptoms such as jaw pain, arthralgia, joint space effusions, diarrhea, encephalitis, pneumonitis, and mediastinal lymphadenopathy and ascites can also be seen. Laboratory findings usually include elevation of the liver injury tests and circulating atypical lymphocytes on the peripheral blood smear. Pancytopenia is also noted in some patients(1-7).
Unresolved and/or recurrent Epstein-Barr viral syndromes often culminate in the development of a posttransplant lymphoproliferative disorder (PTLD)(7, 10-13), which most commonly affects the lymph nodes, hepatic allograft and gastrointestinal tract. However, PTLD can involve any site in the body, where signs and symptoms attributable to a mass lesion are common.
First line therapeutic intervention, regardless of the clonality or histopathological appearance, consists of a dramatic reduction or withdrawal of immunosuppression with the addition of anti-viral agents like acyclovir(7, 10-13). This maneuver is an attempt to restore immune surveillance, which, if successful, can spare the patient from the complications of chemotherapy. Lack of a therapeutic response in some cases necessitates an escalation to either conventional chemotherapy or newer cell-based immunobiological intervention. The latter is aimed at boosting immune surveillance by expanding in vitro, T and NK lymphocytes that normally keep EBV clones under control and then re-injecting these cells into the recipient.
Histopathologic Findings
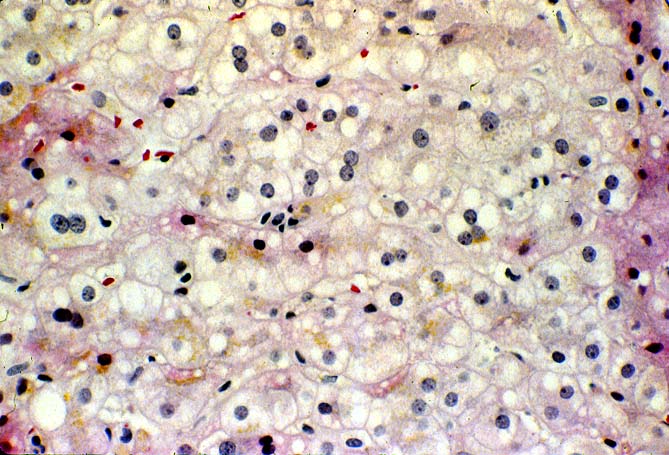
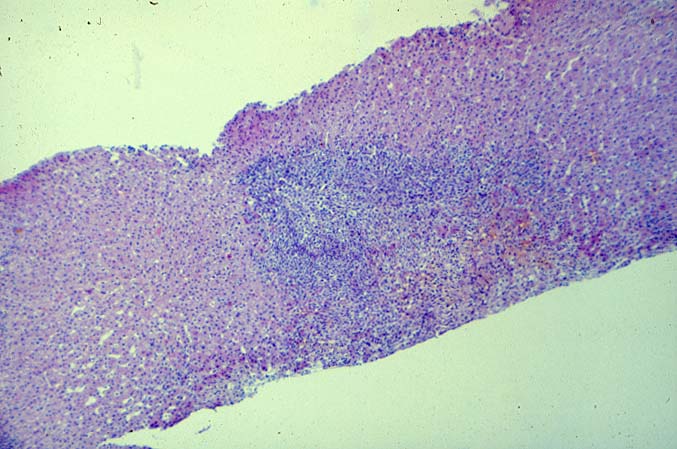
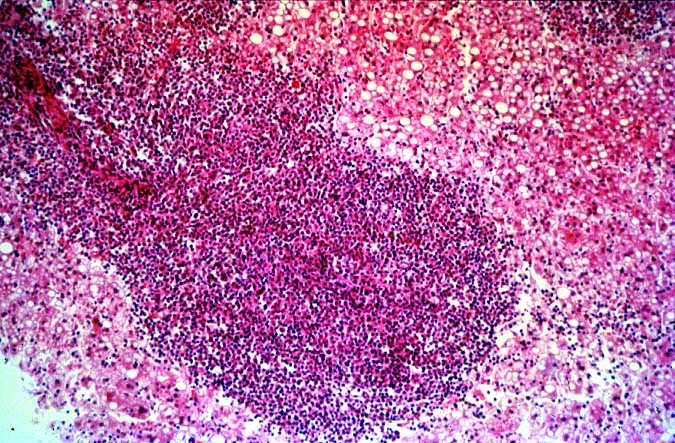
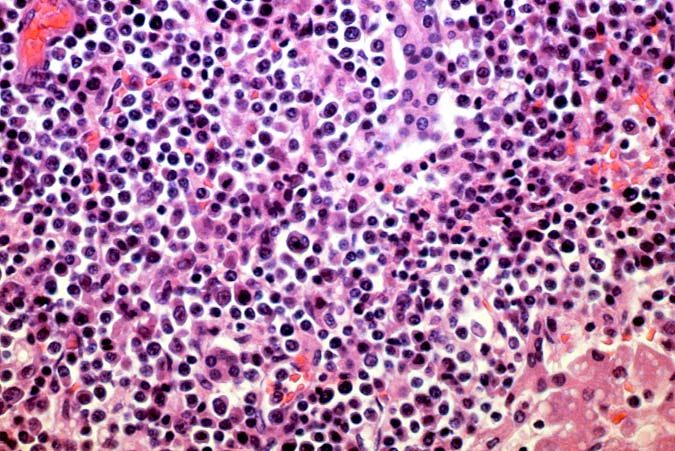
The histopathological findings related to EBV-related disorders in the liver allograft run the gamut from mild EBV hepatitis, like that seen in the general population to hepatic involvement with a post-transplant lymphoproliferative disorder, simulating a malignant lymphoma. EBV hepatitis typically shows features of a "reactive" or low-grade hepatitis with focal hepatocellular swelling, mild acidophilic necrosis of hepatocytes and mild lobular disarray(1-6). Regenerative activity including double-layered plates, pseudoacinar formation and mitotic figures are common. The sinusoids contain linearly aligned and focally aggregated mononuclear cells, cytologically similar to those seen in the portal triads. Occasional granulomatoid Kupffer's cell aggregates can also be seen.
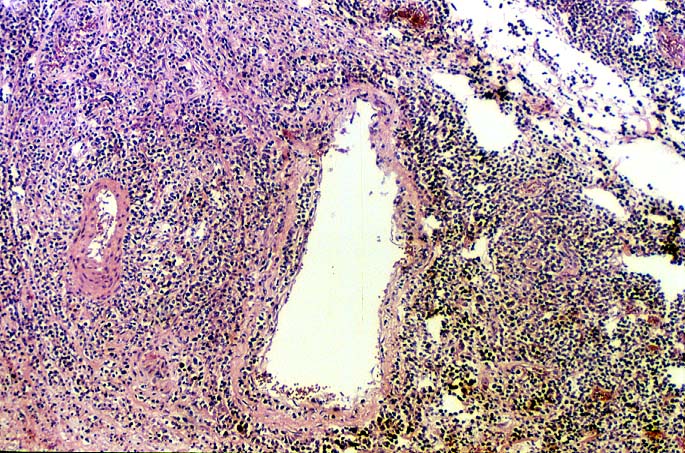
EBV hepatitis usually also shows portal and periportal mononuclear infiltrates of varying severity, comprised of small and blastic lymphocytes, some of which are atypical, admixed with plasmacytoid lymphocytes and plasma cells(1-6). Eosinophils and neutrophils are much less common in the portal tracts than in acute rejection. Bile duct damage can be seen with EBV hepatitis, but the severity and prevalence of duct damage are less than would be expected for rejection, based on the severity of the portal infiltrate(1-6). In addition, EBV hepatitis can show subendothelial localization of lymphocytes in the portal or central vein.
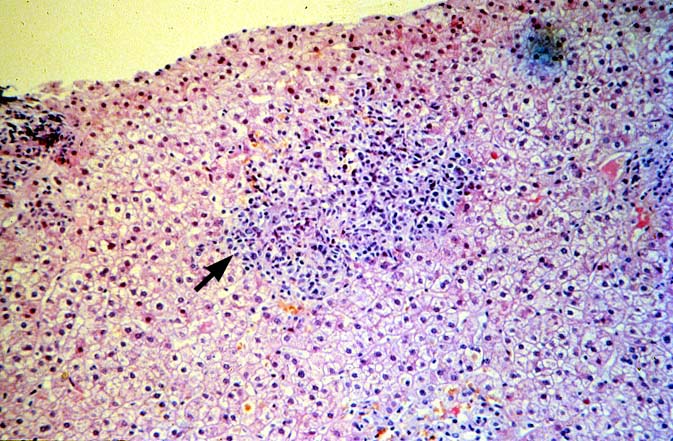
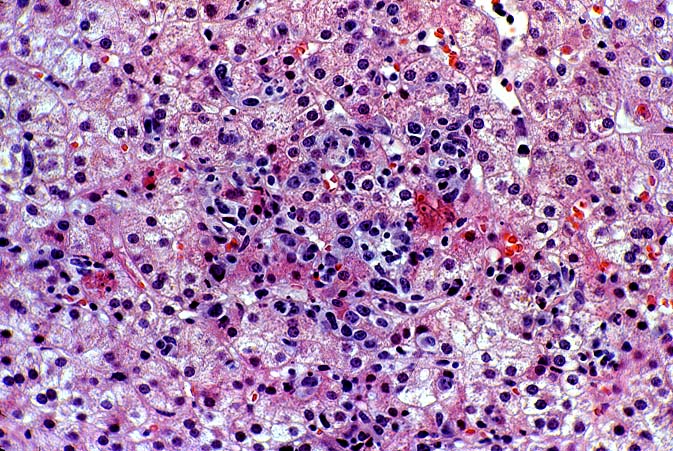
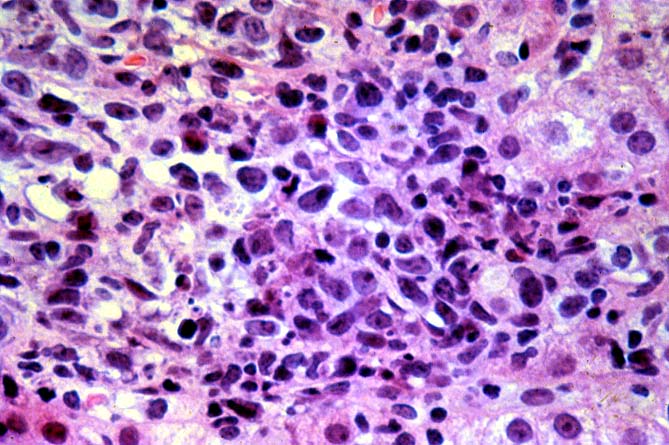
PTLD manifests as map-like enlargement of portal triads because of sheets monomorphic atypical immunoblastic cells. These obscure the normal architectural landmarks and the overall appearance is the same of lymphomatous involvement of the liver(1-6). Smaller aggregates of atypical can also be seen in the sinusoids, and, on occasion, focal areas of necrosis are present. Cytologically , the infiltrate also resembles a malignant lymphoma, with most simulating an immunoblastic lymphoma. On occasion, highly atypical cells, including Reed-Sternberg-like cells can also be seen permeating the sinusoids(14).
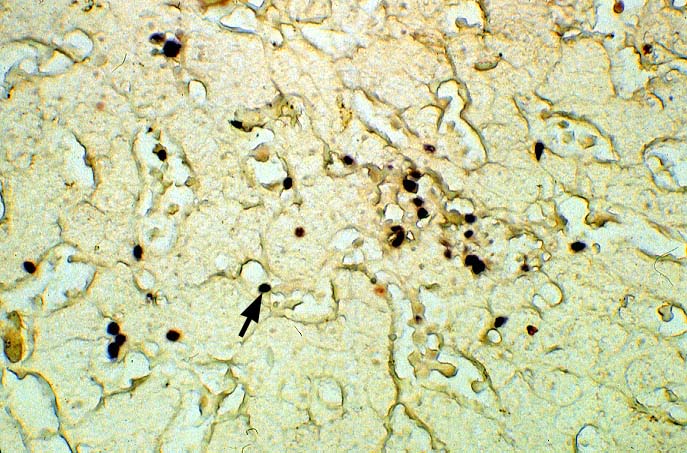
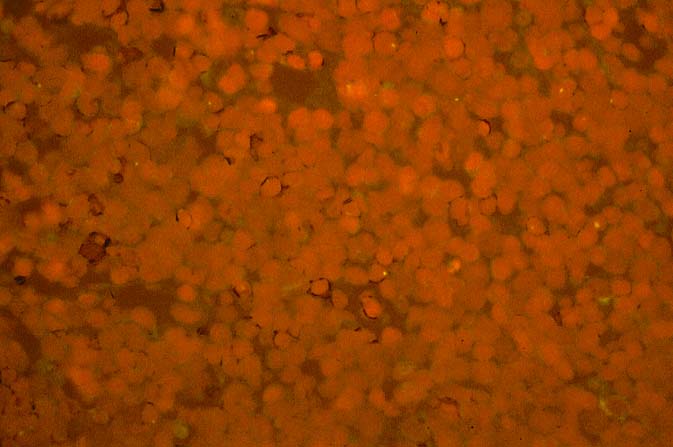
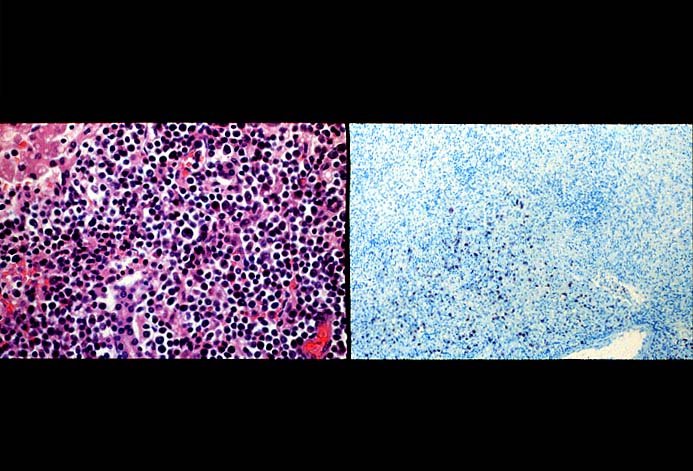
The diagnosis of EBV hepatitis or PTLD is generally suspected on the routine H&E slides and then confirmed by in situ hybridization for EBV RNA (EBER sequence). The reader is referred to the Neoplasia section, for a detailed discussion of the extrahepatic manifestation of PTLD in lymph nodes and other tissues(7, 10-13).
Differential Diagnosis
EBV hepatitis is most often confused with acute severe acute rejection. Depending on the pattern of liver involvement, on occasion, it can also be confused with acute hepatitis types B and C and CMV hepatitis(1-6). Close attention to detailed histopathological findings; clinical correlation and use of adjuvant techniques to identify EBV nucleic acids in the tissue are used to confidently make the distinction in such cases(1-6).
There are several key features on routine light microscopy that assist in distinguishing between EBV hepatitis or PTLD and acute rejection. Although both disorders show predominantly mononuclear portal inflammation, in acute rejection, the portal infiltrate typically is mixed. It contains some blastic and slightly atypical lymphocytes, smaller lymphocytes, neutrophils, plasma cells, and often numerous eosinophils. In contrast, portal infiltrates in EBV hepatitis and PTLD are less pleomorphic, consisting predominantly of activated and immunoblastic atypical mononuclear cells, many of which contain features of plasmacytic differentiation. In addition, eosinophils and neutrophils are less common in EBV-related disorders.
The relationship between the severity of portal inflammation and bile duct damage can also be helpful in distinguishing acute rejection from EBV-related disorders. In acute rejection, inflammatory infiltration and damage of bile ducts is usually widespread and directly proportional to the severity of the portal infiltrate: the greater the portal inflammation, the more severe and widespread the duct damage. In contrast, bile duct damage in EBV-related disorders is relatively mild compared to the intense portal inflammation(1-6).
On occasion, mild EBV hepatitis can be difficult to distinguish from acute viral hepatitis, types B and C and CMV(without inclusions). All of these disorders can show mild lobular disarray, Kupffer's cell hypertrophy, spotty acidophilic necrosis of hepatocytes and sinusoidal lymphocytosis, or a linear alignment of mononuclear cells in the sinusoids. Close attention to detailed histopathological findings, clinical correlation and use of adjuvant test to detect the viruses are needed to make the correct diagnosis. One of the most important points to remember is that EBV-related disorders are frequently associated with atypical lymphocytes in the liver, whereas in hepatitis B and C the atypical lymphocytes are unusual(1-6).
In our experience liberal use of in situ hybridization for the EBER RNA sequence is needed to fully appreciate the range of EBV-related disorders. This technique that has greatly assisted in the management of allograft recipients(1-3, 15) and widespread availability has led to a greater appreciation of the variety of manifestation associated with EBV infection in this population. However, the results of EBER probing on tissue samples from allograft recipients have to be interpreted with caution(15). It is known that occasional EBER+ cells are not uncommon in the general population and are found with increased frequency in allograft recipients. In our experience, the significance of occasional EBER+ cells in this population is open to debate(15), but finding clusters of EBER+ cells, or EBER+ cells in tissues showing other histopathologic features of EBV associated disease, suggest at least a transient defect in the ability to control viral replication and B cell proliferation. The immunosuppression therapy and any signs of EBV-related disease in such patients, should be carefully monitored.
|
|
|
|