The resected left hepatic lobe of the mother was 260 g, i.e. 0.60% of the recipient body weight. Recipient total hepatectomy and grafting procedure followed standard technique. Graft cold and warm ischemia were 146 and 65 minutes, respectively. Intraoperative course and graft reperfusion were excellent, and so was expected postoperatively. the patient showed waking signs in the next morning. However, liver function tests showed progressive deterioration in the first 20 hours after operation; serum bilirubin from 10.8 to 32.1 mg/dl, AST from 151 to 744 IU/I; ALT from 106 to 871 IU/I,; ammonia from 145 to 335 g/dl, and prothrombin time from 38.2 to 49.4 seconds. The patient fell into deep coma again in the afternoon of day 1. Repeated doppler ultrasonography showed sufficient blood flow and no stenosis in all hepatic vessels. Plasma exchange was started in the evening of day 1, with transient and incomplete effectiveness on liver function tests.
On day 2, with further deterioration of laboratory tests, diffuse poor activity on encephalogram aggravated with time. Fulminant graft failure was diagnosed and retransplantation was applied to Ethical Committee according to the offer from father with known possible risk of hepatitis C virus transmission. Emergent retransplantation was done at night of day 2. The explanted first graft was 380 kg and was very hard and patchy with hemorrhagic areas. Review of outside material.
Previous Biopsies on this Patient:
NONE
TPIS Related Resources:
Liver Allograft Rejection Grading
Liver Transplant Topics
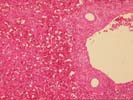


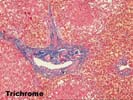

The normal lobular architecture is intact: most of the portal tracts and central veins maintain their normal spacing. However, there is widespread coagulative- type necrosis with marked congestion and hemorrhage. At the border between the necrotic and more viable hepatic parenchyma, the necrosis shows an apparent periportal to mid-zonal pattern, with relative preservation of perivenular hepatocytes.
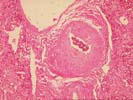
There is no significant portal inflammation, inflammatory duct damage, portal venulitis, endothelial hypertrophy in hepatic artery branches, inflammatory arteritis or other findings suggestive of humeral rejection. However, Section 3-4 does contain a relatively large hepatic artery branch with a recent thrombus. It is difficult to determine whether this was a main anastomotic branch or a vessel tied off at the margin of the resected lobe. The media of the affected vessel shows the degenerative changes. Several other small hepatic arterial branches show mild to moderate fibrointimal hyperplasia. The mild perivenular and pericellular fibrosis seen in the subcapsular pre-transplant wedge biopsy above, is seen in several of the deeper sections. In addition, one section of more viable parenchyma shows mild intra-lobular regenerative change with thickening of the plates. Overall, these mild regenerative changes are similar to those seen with nodular regenerative hyperplasia, but not nearly extensive enough to warrant that diagnosis.
Overall, we are in agreement Dr Yamabe, that the histopathological findings are not supportive of a diagnosis of antibody-mediated rejection. This contention is based on the lack of arterial inflammation, significant arterial endothelial cell hypertrophy, neutrophilic venulitis, inflammation of the peribiliary plexus and negative lymphocytotoxic crossmatch in an ABO-compatible situation. Overall, the most likely cause of liver injury is vascular in nature, but the exact cause(s) of the widespread necrosis cannot be determined with absolute certainty with the information at hand.
First, it should be determined whether the arterial thrombosis detected microscopically was simply an intentionally ligated vessel, or represented a significant arterial supply to the liver fragment. However, as mentioned, the relatively bland, non-inflammatory nature of the necrosis; the areas showing a zonal periportal predominance; the gross appearance of the liver(sharp edges) strongly suggest a "non-immunological" cause. In addition, the mild arterial intimal thickening, the perivenular and pericellular fibrosis, along with the mild intralobular regenerative change suggests some mild underlying abnormalities in the donor.
I have seen a non-hemorrhagic, periportal to midzonal pattern of necrosis in several other patients with an inadequate portal vein blood supply. In fact, a porta-caval shunt in a patient with a normal liver will cause a pattern of injury similar to that seen in this particular case(I am currently preparing a case report). Therefore, I would think it is reasonable to assume that hyperperfusion of the portal vein could result in a periportal to midzonal pattern of hemorrhagic necrosis, like that seen in this case. The mild underlying abnormalities in the donor liver may have contributed to the hemodynamic problems encountered. I look forward to any follow-up in this case.