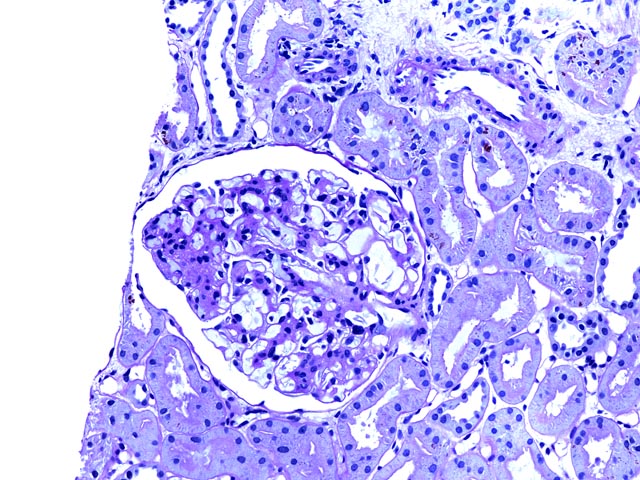
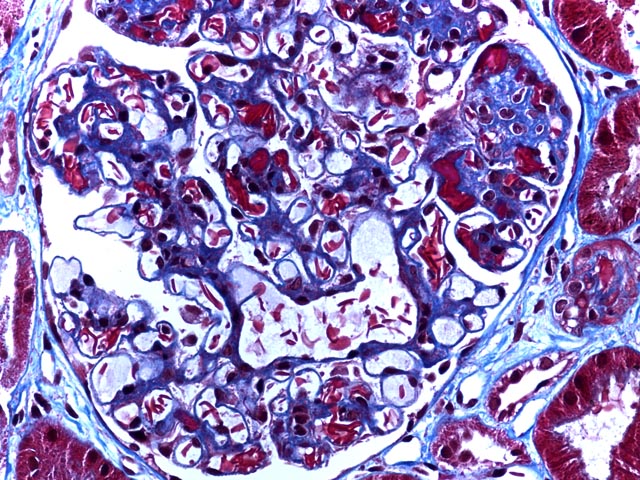
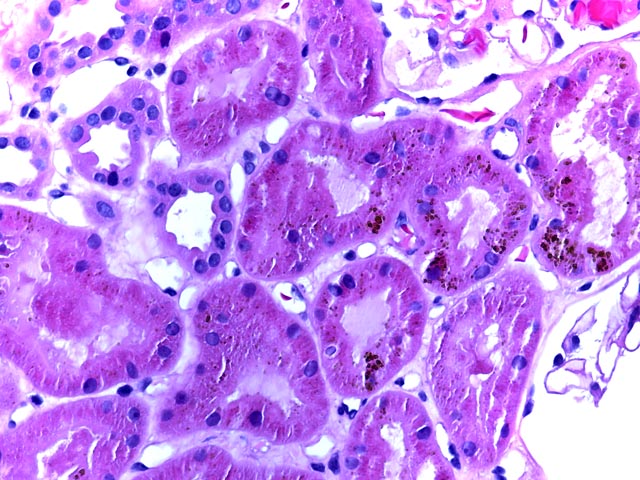
This young man had prior episodes of sickle cell crisis following his transplant. Biopsy showed sickled erythrocytes with diffuse glomerular capillary congestion and mesangial widening. Hemosiderin deposition was seen in tubules, but neither tubular necrosis nor significant acute rejection were identified. His symtpoms resolved and he continues to do well with a functioning allgoraft.
Figure 1 is a PAS stain (200x) showing an intact glomerulus with mesangial widening due to an increase in matrix and cellularity. Adjacent tubules show no evidence of rejection, and some pigment within tubular epithelial cells represents hemosiderin.
Figure 2 is a trichrome stain (400x) again showing increased mesangium, but highlighting the sickled erythrocytes within glomerular capillary lumens. Areas of erythrocyte congestion and sludging are also apparent.
Figure 3 is an H&E stain (400x) highlighting the presence of hemosiderin and showing a lack of inflammation. Biopsy is an important means of distinguishing between rejection and allograft dysfunction due to underlying sickle cell disease.
Background: Sickle Cell Disease in Native Kidney
Erythrocyte sickling with subsequent intravascular sludging is associated with several pathologic alterations in the native kidney. Arteriolar and capillary congestion may occur. Hemosiderin deposition in tubular epithelial cells occurs, and injury to these cells may lead to episodes of necrosis with regeneration and pigment casts. Interstitial fibrosis and tubular atrophy occur with disease progression. Significant involvement of the vasa recta leads to microinfarcts that may coalesce to lead to more prominent medullary injury, and ultimately in some cases to renal papillary necrosis.
Glomerular capillary congestion also occurs and may be prominent. Focal segmental glomerulosclerosis and membranoproliferative patterns of glomerulonephritis are among the more commonly described glomerular alterations. Although glomerular involvement is most commonly related to underlying sickle cell disorder, unrelated glomerulopathies can of course occur and renal biopsy is useful in distinguishing among these possibilities.
An additional complication that occurs almost exclusively in African Americans with sickle cell trait is renal medullary carcinoma.
This presentation does not address therapy or management of sickle cell disease except for considering some pathologic aspects of the postransplant state. Several representative recent reviews address current and evolving therapies (5, 7-9, 13).
Renal Transplantation in Sickle Cell Disease
Outcomes
In an early study, Chaterjee et al. (3) reviewed data from 18 transplant centers that transplanted 34 kidneys into 30 patients with sickle cell disease or trait. The authors concluded that patient and graft survivals were comparable to other renal transplant recipients. This conclusion extends to the pediatric population according to Warady et al. (18), who examined the data of the North American pediatric renal transplant cooperative study. They found 9 pediatric patients who received renal transplants between the years 1989 and 1995. Twelve and 24 month graft survival was 89% and 71%, with 89% patient survival.
Abbott et al. (1) found that patients with sickle cell disease constituted 0.11% of patients treated for end-stage renal disease in the U.S. between 1992-June 1997. These patients were less likely to undergo transplant compared to age and race matched controls.
Complications
In addition to the usual risks associated with the immunosuppressed posttransplant state, renal transplant patients with sickle cell disease are at specific risk for complications including sickle cell crises and recurrent disease. Potentially increased risks for other complications such as propensity for infection in patients with splenic autoinfarction and atrophy, or aseptic necrosis related to steroid therapy, have also been suggested.
Sickle cell crises
Increased frequency of sickle cell crisis is a potential complication generally considered to be related to posttransplant increase in hemoglobin/hematocrit levels and plasma viscosity. In the study of Chaterjee et al. (3), 8 of 30 patients had sickle cell crises in the first posttransplant year. Fischer et al. (6) presented a case of a patient who also developed recurrent episodes of deep vein thrombosis in addition to such painful crises following renal transplant.
Myers et al. (12) reported increased frequency of crises in a patient who originally developed renal failure not from the direct effects of sickle cell anemia, but from a concurrent pheochromocytoma. Nevertheless, this posttransplant complication was also associated with increased hemoglobin concentration and was due to underlying sickle cell disease.
Recurrent renal disease
The frequency with which disease recurrence leads to sickle cell nephropathy in the allograft is not well documented. Miner et al. (11) report a case of a patient who developed a permanent decline in renal function, 3 1/2 years after transplant due to recurrence of sickle cell nephropathy and Chaterjee et al. (4) provided another early case report of post transplant renal allograft dysfunction that was not reversible by immunosuppression and was related to intravascular sickling. O’Rourke et al. (14) recently presented a radiographic teaching case in which recurrent sickle cell involvement of the allograft presented as acute dysfunction, with biopsy findings of vascular congestion and patchy cortical necrosis.
Other Topics
Multiple organ transplants
Several reports have shown the feasibility of multiorgan transplantation in sickle cell disease in the appropriate setting. Ross et al. (17) described a patient who received kidney and liver transplantation for relief of both sickle cell related end-stage renal disease and acute sickle cell intrahepatic cholestasis. The patient survived for 22 months at which time he expired due to bilateral hip fractures leading to acute pulmonary embolism. Neither rejection nor recurrent disease was seen in either organ and the authors concluded that, despite patient outcome, this represented a viable option in sickle cell patients with multiorgan disease. Audard et al. (2) reported a young adult male with underlying homozygous sickle cell disease who underwent combined heart and kidney transplantation. The patient was alive with stable allograft function 18 months after transplant.
Pregnancy in Recipients with Sickle Cell Disease
Mallat et al. (10) and Westney et al. (19) reported individual cases of successful pregnancies in patients who have had sickle cell disease and renal transplantation.
Donors with sickle cell disease
Reese et al. (15) surveyed U. S. transplant centers and found that, of the respondents, only 34% screened donors for sickle trait. 37% reported excluding donors with sickle trait always or most of the time. It would appear that there is a need for some guidance in this area.
Rehman et al. (16) raise the issue of donors with sickle cell trait in relation to the concern that the kidneys may not concentrate urine properly. They report an example of a living related renal transplant in which both donor and recipient had sickle cell trait. No complications were seen following the transplant in either donor or recipient.
References
1. Abbott KC, Hypolite IO, Agodoa LY. Sickle cell nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 2002;58:9-15.
2. Audard V, Grimbert P, Kirsch M, et al. Successful combined heart and kidney transplantation in a patient with sickle-cell anemia. J Heart Lung Transplant 2006;25:993-6.
3. Chatterjee SN. National study on natural history of renal allografts in sickle cell disease or trait. Nephron 1980;25:199-201.
4. Chatterjee SN, Lundberg GD, Berne TV. Sickle cell trait: possible contributory cause of renal allograft failure. Urology 1978;11:266-8.
5. de Santis Feltran L, de Abreu Carvalhaes JT, Sesso R. Renal complications of sickle cell disease: managing for optimal outcomes. Paediatr Drugs 2002;4:29-36.
6. Fischer GB, da Rosa AC. Thrombosis after kidney transplantation. Blood Coagul Fibrinolysis 2009;20:456-7.
7. Hankins J, Aygun B. Pharmacotherapy in sickle cell disease--state of the art and future prospects. Br J Haematol 2009;145:296-308.
8. Haywood C, Jr., Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc 2009;101:1022-33.
9. Kotiah SD, Ballas SK. Investigational drugs in sickle cell anemia. Expert Opin Investig Drugs 2009;18:1817-28.
10. Mallat SG, Brensilver JM, McCabe R, Nurse HM. Successful pregnancy in a cyclosporine-treated renal transplant recipient with sickle cell disease. Transplantation 1988;45:660-1.
11. Miner DJ, Jorkasky DK, Perloff LJ, Grossman RA, Tomaszewski JE. Recurrent sickle cell nephropathy in a transplanted kidney. Am J Kidney Dis 1987;10:306-13.
12. Myers B, Donohue SM. A case of sickle-cell erythrocytosis occurring following renal transplantation. Clin Lab Haematol 2002;24:175-7.
13. Nagalla S, Ballas SK. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Syst Rev 2010:CD003426.
14. O'Rourke EJ, Laing CM, Khan AU, et al. The case. Allograft dysfunction in a patient with sickle cell disease. Kidney Int 2008;74:1219-20.
15. Reese PP, Hoo AC, Magee CC. Screening for sickle trait among potential live kidney donors: policies and practices in US transplant centers. Transpl Int 2008;21:328-31.
16. Rehman S, Al-Amoudi A, Kelta M, Awad A, Bridges K, Al-Ghamdi SM. Kidney transplant from sickle cell trait donor to sickle cell trait recipient. Exp Clin Transplant 2007;5:698-700.
17. Ross AS, Graeme-Cook F, Cosimi AB, Chung RT. Combined liver and kidney transplantation in a patient with sickle cell disease. Transplantation 2002;73:605-8.
18. Warady BA, Sullivan EK. Renal transplantation in children with sickle cell disease: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Transplant 1998;2:130-3.
19. Westney LS, Callender CO, Stevens J, Bhagwanani SG, George JP, Mims OL. Successful pregnancy with sickle cell disease and renal transplantation. Obstet Gynecol 1984;63:752-5.