|
Recurrence of Diabetes
Mellitus Type I 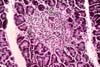
Recurrence of Type I diabetes mellitus has
been well documented in approximately a
dozen cases. The diagnosis of recurrent disease is made with the combination of
sudden or progressive lack of glycemic
control associated with selective loss of beta cells in the graft and
persistence of the other types of islet cells, particularly alpha cells. In few
cases the active phase of beta cell
destruction has been demonstrated; this consists of selective mononuclear cell infiltration (isletitis). The
inflammation is centered in islets still containing beta cells. The isletitis
resolves when beta cells disappear. The majority of documented cases of recurrent
diabetes mellitus occurred in transplants from identical twins or HLA-identical
siblings. In some cases isletitis
resolved after introduction or increase in immunosuppression. HLA-mismatched
transplants from cadaveric donors are, however, also susceptible to selective
beta cells destruction. The latter appears to result from insufficient
immunosuppression. In addition to the
clinical and histological findings, the diagnosis of recurrent autoimmune
disease is aided by the demonstration of islet cell autoantibodies in serum
(GAD 65 and IA-2). In a study comparing patients with chronic graft failure versus patients with well
functioning grafts, islet cell autoantibodies before transplantation and at the
time of graft failure were significantly higher in the former group. Also, patients with failed grafts showed an
increase in autoantibodies at the time of loss of graft function. Recurrent autoimmunity appears to operate
also in the case of allogeneic islet transplantation. The rarity of recurrent Type I diabetes
mellitus in whole pancreas transplantation has been attributed to the inclusion
of donor lymphoid tissue with the transplanted pancreas. This would lead to
recipient chimerism for a donor T cell subset (RT6.2). We have not observed isletitis or selective
beta cell loss after routinely evaluating immunoperoxidase stains for insulin
and glucagon in over 600 pancreas transplant biopsies and over a hundred
pancreatectomies. We have not been able to demonstrate islet cell
autoantibodies in a small number with clinical presentation suspicious for
recurrent diabetes mellitus. It is important to emphasize that
non-specific inflammation of islets and with no associated selective loss of
beta cells can be seen in acute allograft rejection. Islet inflammation is
proportional to the degree of inflammation in the neighboring exocrine
parenchyma and usually consists of a mixture of inflammatory cells that may
include eosinophils. (Pictures: isletitis, inflammation in rejection normal pattern of beta
and alpha cells in islets) Histological correlation of possible causes of hyperglycemia in
pancreas transplant patients: Severe acute rejection: extensive parenchymal inflammation and or
necrosis that affects both exocrine and endocrine component. Thrombosis of large vessels: extensive parenchymal inflammation and or
necrosis that affects both exocrine and endocrine component. Chronic rejection: Progressive
graft fibrosis is associated with loss of glycemic control. The graft looks
sclerotic and there is extensive acinar loss. Residual islets contain beta cells. Drug toxicity: The islet cells show vacuolization and swelling. The acinar component is normal (both acinar
and endocrine components are vacuolized in ischemic injury). Recurrence of autoimmune diabetes: Active destructive phase shows
isletitis with progressive and selective loss of beta cells. The acinar
component is not affected. In the
inactive phase (after the disappearance of beta cells) the pancreas will look superficially normal.
In these cases the loss of beta cells can be demonstrated by the lack of
insulin stain in islets. After a
prolonged period of time the islets as a whole can disappear.
References:

Please mail comments, corrections or suggestions to the TPIS administration at the UPMC.
If you have more questions, you can always email TPIS Administration. |
|